Daniel H. Pak
Snap-and-tune: combining deep learning and test-time optimization for high-fidelity cardiovascular volumetric meshing
Jun 09, 2025Abstract:High-quality volumetric meshing from medical images is a key bottleneck for physics-based simulations in personalized medicine. For volumetric meshing of complex medical structures, recent studies have often utilized deep learning (DL)-based template deformation approaches to enable fast test-time generation with high spatial accuracy. However, these approaches still exhibit limitations, such as limited flexibility at high-curvature areas and unrealistic inter-part distances. In this study, we introduce a simple yet effective snap-and-tune strategy that sequentially applies DL and test-time optimization, which combines fast initial shape fitting with more detailed sample-specific mesh corrections. Our method provides significant improvements in both spatial accuracy and mesh quality, while being fully automated and requiring no additional training labels. Finally, we demonstrate the versatility and usefulness of our newly generated meshes via solid mechanics simulations in two different software platforms. Our code is available at https://github.com/danpak94/Deep-Cardiac-Volumetric-Mesh.
Progressive Test Time Energy Adaptation for Medical Image Segmentation
Mar 20, 2025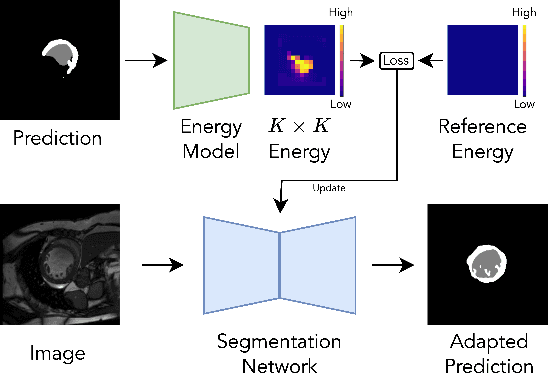
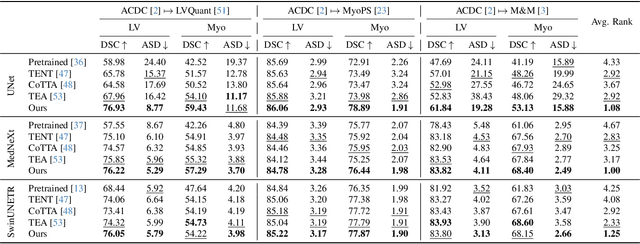
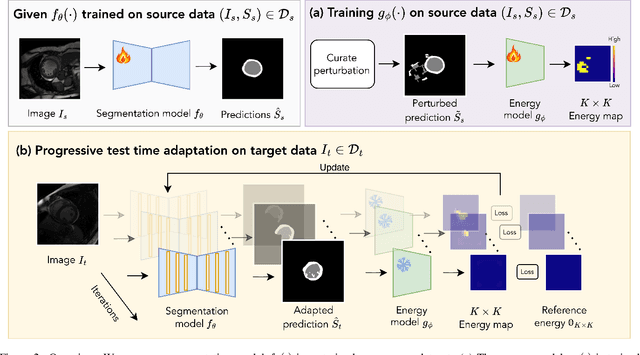
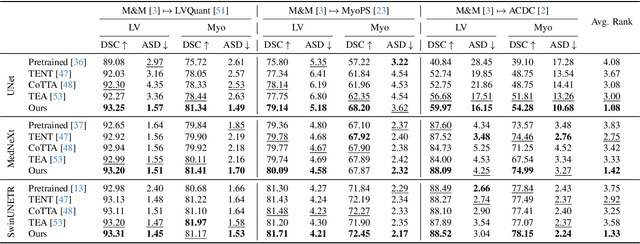
Abstract:We propose a model-agnostic, progressive test-time energy adaptation approach for medical image segmentation. Maintaining model performance across diverse medical datasets is challenging, as distribution shifts arise from inconsistent imaging protocols and patient variations. Unlike domain adaptation methods that require multiple passes through target data - impractical in clinical settings - our approach adapts pretrained models progressively as they process test data. Our method leverages a shape energy model trained on source data, which assigns an energy score at the patch level to segmentation maps: low energy represents in-distribution (accurate) shapes, while high energy signals out-of-distribution (erroneous) predictions. By minimizing this energy score at test time, we refine the segmentation model to align with the target distribution. To validate the effectiveness and adaptability, we evaluated our framework on eight public MRI (bSSFP, T1- and T2-weighted) and X-ray datasets spanning cardiac, spinal cord, and lung segmentation. We consistently outperform baselines both quantitatively and qualitatively.
AI-powered multimodal modeling of personalized hemodynamics in aortic stenosis
Jun 29, 2024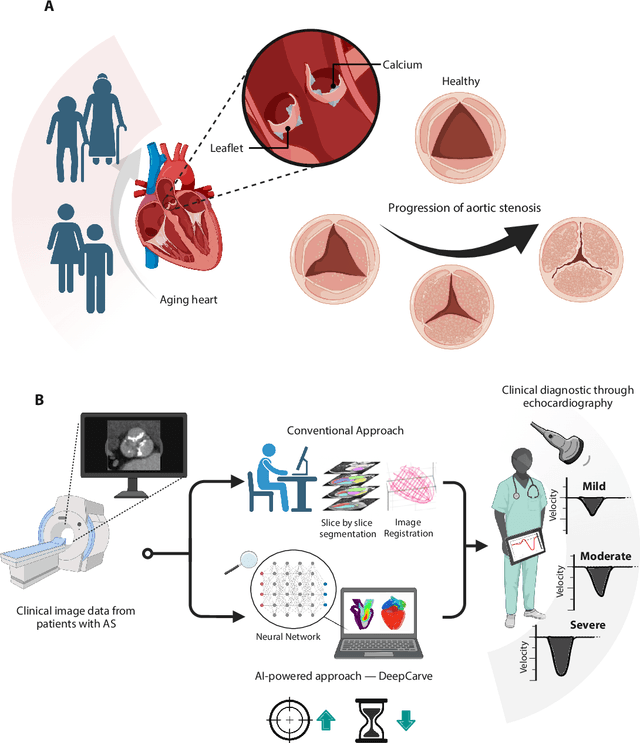
Abstract:Aortic stenosis (AS) is the most common valvular heart disease in developed countries. High-fidelity preclinical models can improve AS management by enabling therapeutic innovation, early diagnosis, and tailored treatment planning. However, their use is currently limited by complex workflows necessitating lengthy expert-driven manual operations. Here, we propose an AI-powered computational framework for accelerated and democratized patient-specific modeling of AS hemodynamics from computed tomography. First, we demonstrate that our automated meshing algorithms can generate task-ready geometries for both computational and benchtop simulations with higher accuracy and 100 times faster than existing approaches. Then, we show that our approach can be integrated with fluid-structure interaction and soft robotics models to accurately recapitulate a broad spectrum of clinical hemodynamic measurements of diverse AS patients. The efficiency and reliability of these algorithms make them an ideal complementary tool for personalized high-fidelity modeling of AS biomechanics, hemodynamics, and treatment planning.
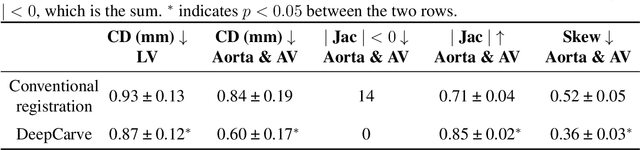
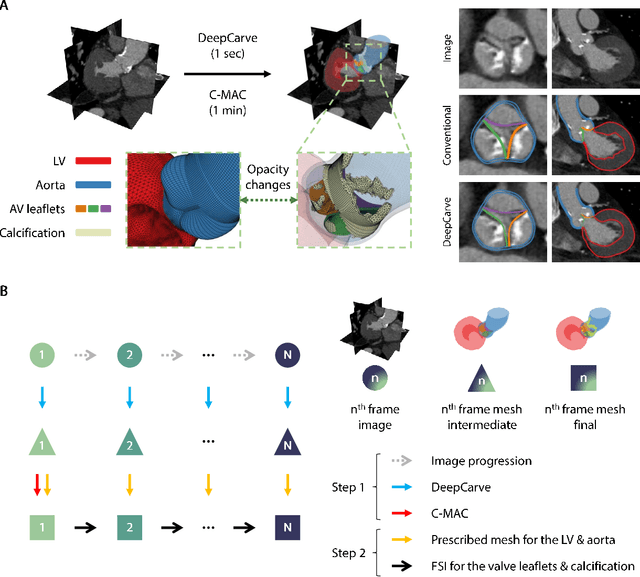
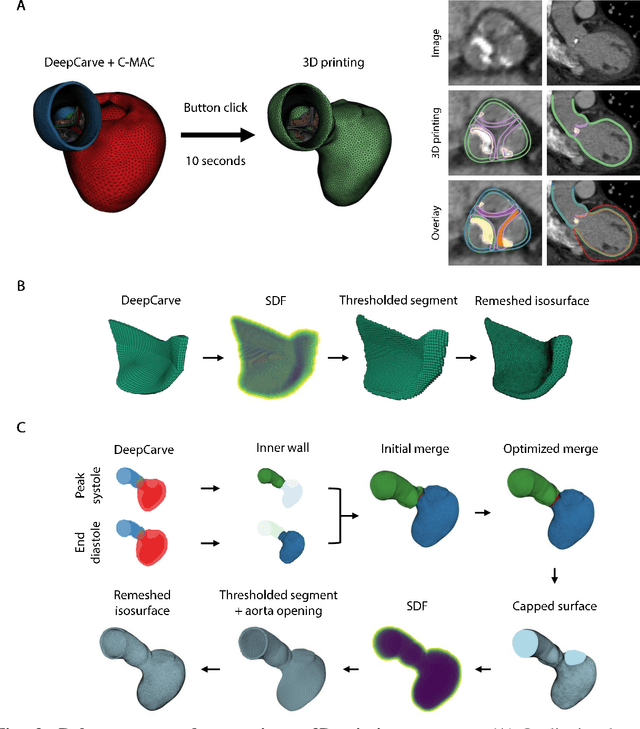
Robust automated calcification meshing for biomechanical cardiac digital twins
Mar 08, 2024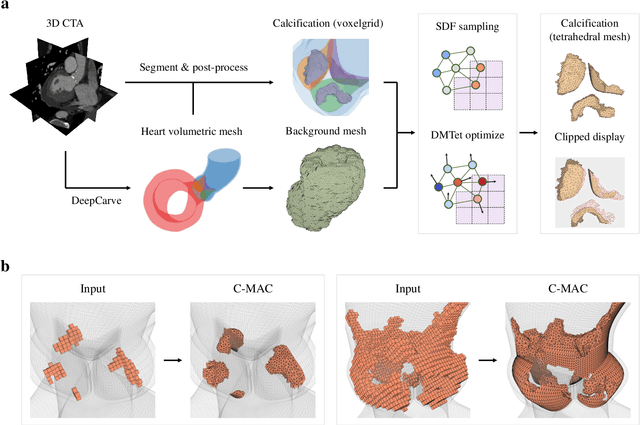
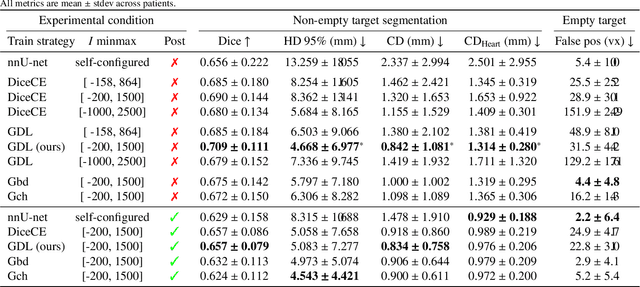
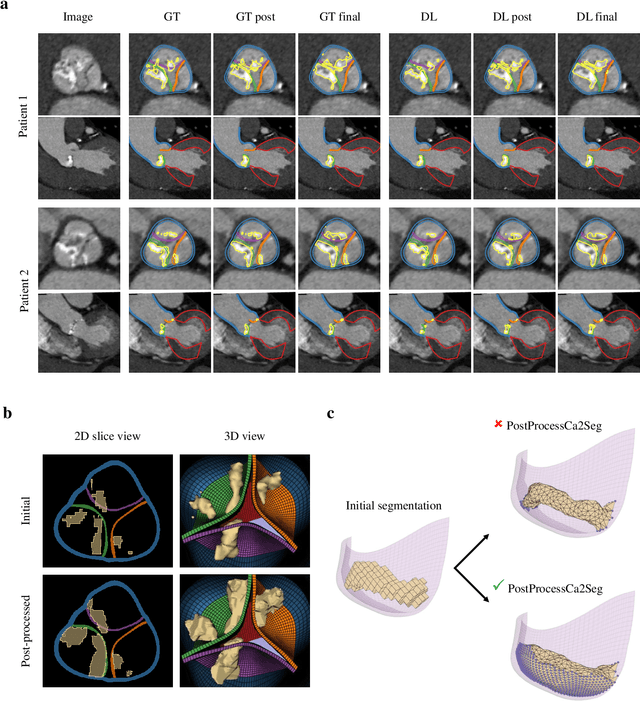
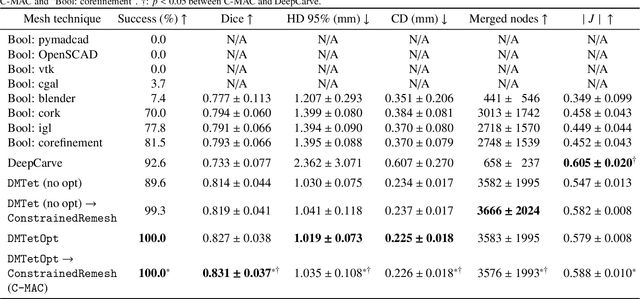
Abstract:Calcification has significant influence over cardiovascular diseases and interventions. Detailed characterization of calcification is thus desired for predictive modeling, but calcified heart meshes for physics-driven simulations are still often reconstructed using manual operations. This poses a major bottleneck for large-scale adoption of computational simulations for research or clinical use. To address this, we propose an end-to-end automated meshing algorithm that enables robust incorporation of patient-specific calcification onto a given heart mesh. The algorithm provides a substantial speed-up from several hours of manual meshing to $\sim$1 minute of automated computation, and it solves an important problem that cannot be addressed with recent template registration-based heart meshing techniques. We validated our final calcified heart meshes with extensive simulations, demonstrating our ability to accurately model patient-specific aortic stenosis and Transcatheter Aortic Valve Replacement. Our method may serve as an important tool for accelerating the development and usage of physics-driven simulations for cardiac digital twins.
Heteroscedastic Uncertainty Estimation for Probabilistic Unsupervised Registration of Noisy Medical Images
Dec 01, 2023



Abstract:This paper proposes a heteroscedastic uncertainty estimation framework for unsupervised medical image registration. Existing methods rely on objectives (e.g. mean-squared error) that assume a uniform noise level across the image, disregarding the heteroscedastic and input-dependent characteristics of noise distribution in real-world medical images. This further introduces noisy gradients due to undesired penalization on outliers, causing unnatural deformation and performance degradation. To mitigate this, we propose an adaptive weighting scheme with a relative $\gamma$-exponentiated signal-to-noise ratio (SNR) for the displacement estimator after modeling the heteroscedastic noise using a separate variance estimator to prevent the model from being driven away by spurious gradients from error residuals, leading to more accurate displacement estimation. To illustrate the versatility and effectiveness of the proposed method, we tested our framework on two representative registration architectures across three medical image datasets. Our proposed framework consistently outperforms other baselines both quantitatively and qualitatively while also providing accurate and sensible uncertainty measures. Paired t-tests show that our improvements in registration accuracy are statistically significant. The code will be publicly available at \url{https://voldemort108x.github.io/hetero_uncertainty/}.
Holistic Multi-Slice Framework for Dynamic Simultaneous Multi-Slice MRI Reconstruction
Jan 03, 2023Abstract:Dynamic Magnetic Resonance Imaging (dMRI) is widely used to assess various cardiac conditions such as cardiac motion and blood flow. To accelerate MR acquisition, techniques such as undersampling and Simultaneous Multi-Slice (SMS) are often used. Special reconstruction algorithms are needed to reconstruct multiple SMS image slices from the entangled information. Deep learning (DL)-based methods have shown promising results for single-slice MR reconstruction, but the addition of SMS acceleration raises unique challenges due to the composite k-space signals and the resulting images with strong inter-slice artifacts. Furthermore, many dMRI applications lack sufficient data for training reconstruction neural networks. In this study, we propose a novel DL-based framework for dynamic SMS reconstruction. Our main contributions are 1) a combination of data transformation steps and network design that effectively leverages the unique characteristics of undersampled dynamic SMS data, and 2) an MR physics-guided transfer learning strategy that addresses the data scarcity issue. Thorough comparisons with multiple baseline methods illustrate the strengths of our proposed methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge