Ellen T. Roche
AI-powered multimodal modeling of personalized hemodynamics in aortic stenosis
Jun 29, 2024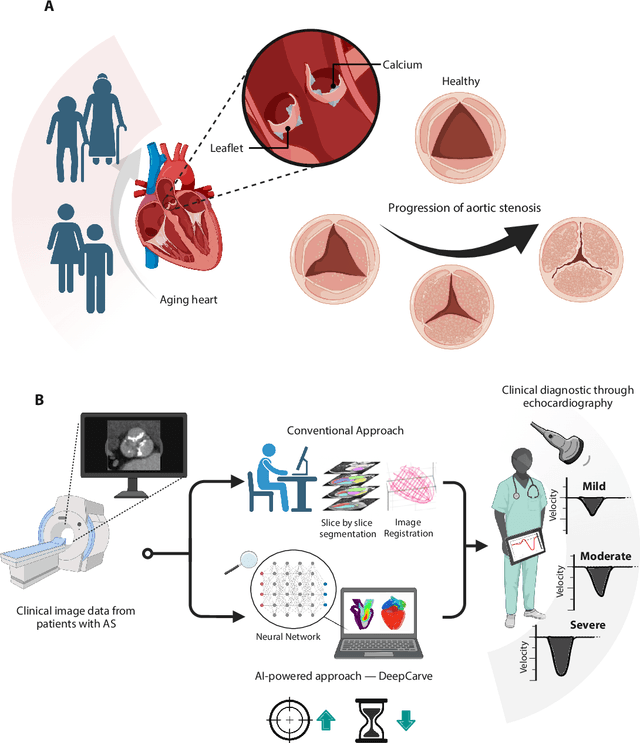
Abstract:Aortic stenosis (AS) is the most common valvular heart disease in developed countries. High-fidelity preclinical models can improve AS management by enabling therapeutic innovation, early diagnosis, and tailored treatment planning. However, their use is currently limited by complex workflows necessitating lengthy expert-driven manual operations. Here, we propose an AI-powered computational framework for accelerated and democratized patient-specific modeling of AS hemodynamics from computed tomography. First, we demonstrate that our automated meshing algorithms can generate task-ready geometries for both computational and benchtop simulations with higher accuracy and 100 times faster than existing approaches. Then, we show that our approach can be integrated with fluid-structure interaction and soft robotics models to accurately recapitulate a broad spectrum of clinical hemodynamic measurements of diverse AS patients. The efficiency and reliability of these algorithms make them an ideal complementary tool for personalized high-fidelity modeling of AS biomechanics, hemodynamics, and treatment planning.
Robust automated calcification meshing for biomechanical cardiac digital twins
Mar 08, 2024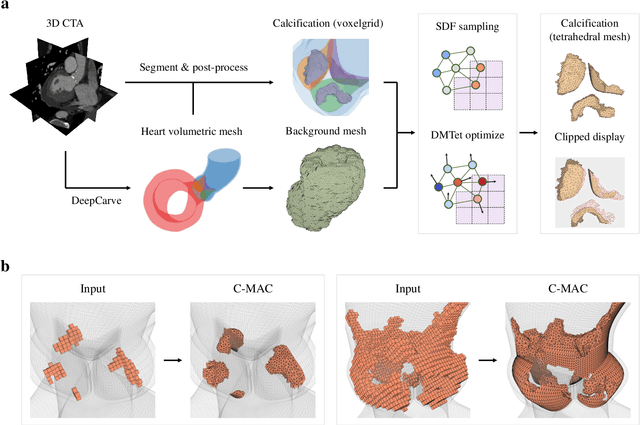
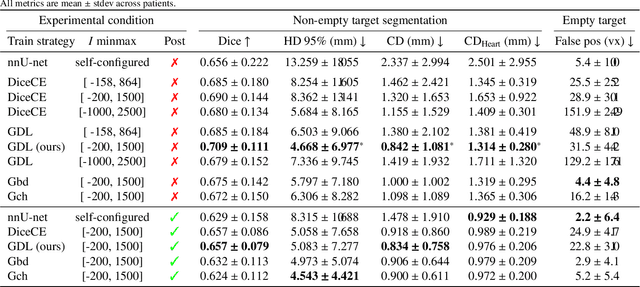
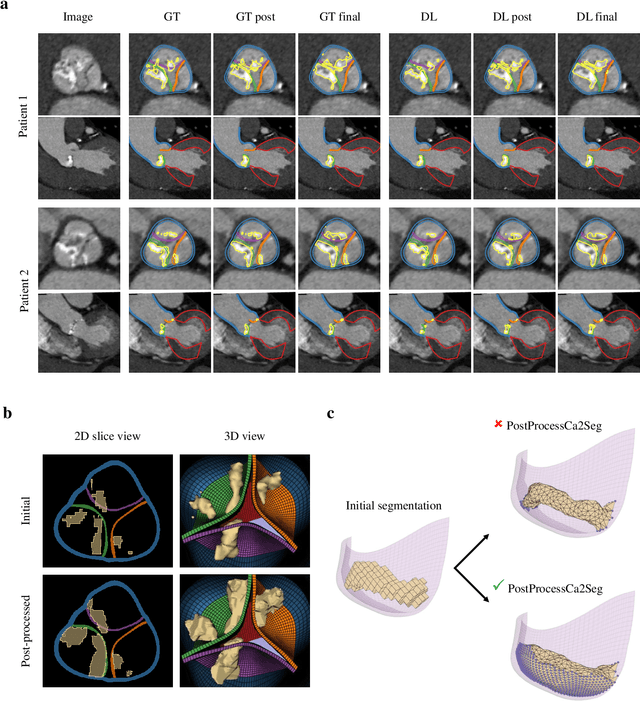
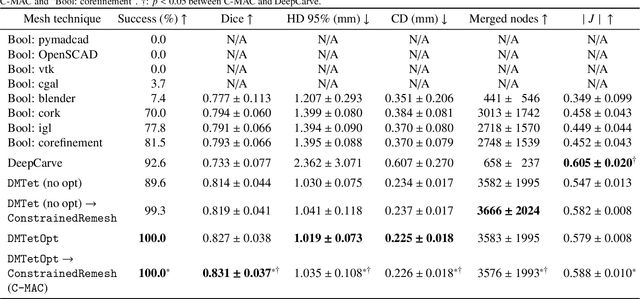
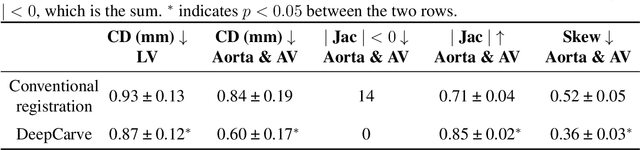
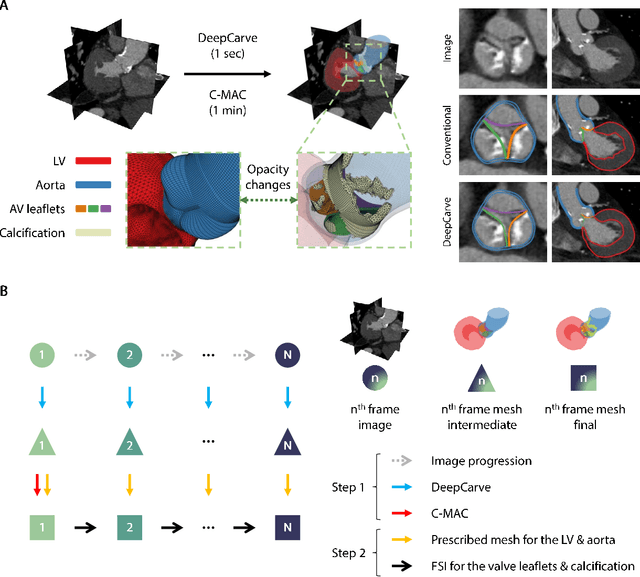
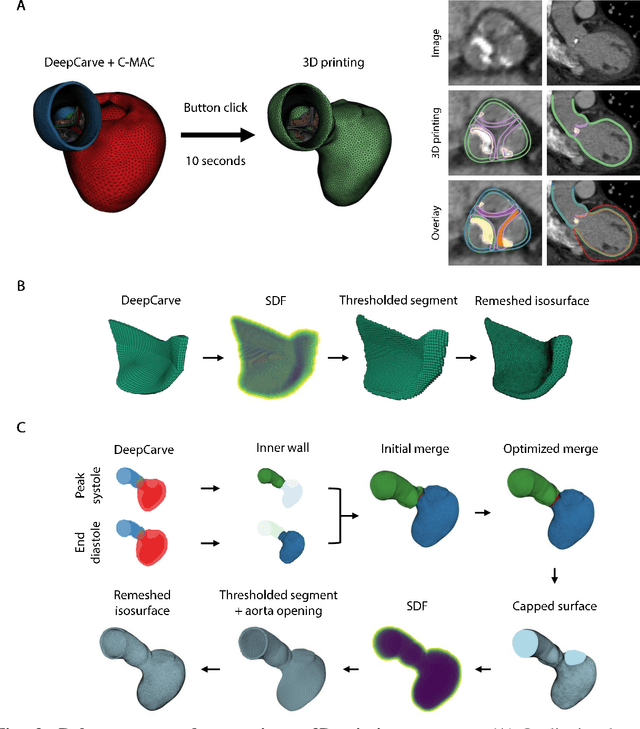
Abstract:Calcification has significant influence over cardiovascular diseases and interventions. Detailed characterization of calcification is thus desired for predictive modeling, but calcified heart meshes for physics-driven simulations are still often reconstructed using manual operations. This poses a major bottleneck for large-scale adoption of computational simulations for research or clinical use. To address this, we propose an end-to-end automated meshing algorithm that enables robust incorporation of patient-specific calcification onto a given heart mesh. The algorithm provides a substantial speed-up from several hours of manual meshing to $\sim$1 minute of automated computation, and it solves an important problem that cannot be addressed with recent template registration-based heart meshing techniques. We validated our final calcified heart meshes with extensive simulations, demonstrating our ability to accurately model patient-specific aortic stenosis and Transcatheter Aortic Valve Replacement. Our method may serve as an important tool for accelerating the development and usage of physics-driven simulations for cardiac digital twins.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge