Bokai Zhang
Friends Across Time: Multi-Scale Action Segmentation Transformer for Surgical Phase Recognition
Jan 22, 2024



Abstract:Automatic surgical phase recognition is a core technology for modern operating rooms and online surgical video assessment platforms. Current state-of-the-art methods use both spatial and temporal information to tackle the surgical phase recognition task. Building on this idea, we propose the Multi-Scale Action Segmentation Transformer (MS-AST) for offline surgical phase recognition and the Multi-Scale Action Segmentation Causal Transformer (MS-ASCT) for online surgical phase recognition. We use ResNet50 or EfficientNetV2-M for spatial feature extraction. Our MS-AST and MS-ASCT can model temporal information at different scales with multi-scale temporal self-attention and multi-scale temporal cross-attention, which enhances the capture of temporal relationships between frames and segments. We demonstrate that our method can achieve 95.26% and 96.15% accuracy on the Cholec80 dataset for online and offline surgical phase recognition, respectively, which achieves new state-of-the-art results. Our method can also achieve state-of-the-art results on non-medical datasets in the video action segmentation domain.
SF-TMN: SlowFast Temporal Modeling Network for Surgical Phase Recognition
Jun 15, 2023Abstract:Automatic surgical phase recognition is one of the key technologies to support Video-Based Assessment (VBA) systems for surgical education. Utilizing temporal information is crucial for surgical phase recognition, hence various recent approaches extract frame-level features to conduct full video temporal modeling. For better temporal modeling, we propose SlowFast Temporal Modeling Network (SF-TMN) for surgical phase recognition that can not only achieve frame-level full video temporal modeling but also achieve segment-level full video temporal modeling. We employ a feature extraction network, pre-trained on the target dataset, to extract features from video frames as the training data for SF-TMN. The Slow Path in SF-TMN utilizes all frame features for frame temporal modeling. The Fast Path in SF-TMN utilizes segment-level features summarized from frame features for segment temporal modeling. The proposed paradigm is flexible regarding the choice of temporal modeling networks. We explore MS-TCN and ASFormer models as temporal modeling networks and experiment with multiple combination strategies for Slow and Fast Paths. We evaluate SF-TMN on Cholec80 surgical phase recognition task and demonstrate that SF-TMN can achieve state-of-the-art results on all considered metrics. SF-TMN with ASFormer backbone outperforms the state-of-the-art Not End-to-End(TCN) method by 2.6% in accuracy and 7.4% in the Jaccard score. We also evaluate SF-TMN on action segmentation datasets including 50salads, GTEA, and Breakfast, and achieve state-of-the-art results. The improvement in the results shows that combining temporal information from both frame level and segment level by refining outputs with temporal refinement stages is beneficial for the temporal modeling of surgical phases.
Biomedical image analysis competitions: The state of current participation practice
Dec 16, 2022Abstract:The number of international benchmarking competitions is steadily increasing in various fields of machine learning (ML) research and practice. So far, however, little is known about the common practice as well as bottlenecks faced by the community in tackling the research questions posed. To shed light on the status quo of algorithm development in the specific field of biomedical imaging analysis, we designed an international survey that was issued to all participants of challenges conducted in conjunction with the IEEE ISBI 2021 and MICCAI 2021 conferences (80 competitions in total). The survey covered participants' expertise and working environments, their chosen strategies, as well as algorithm characteristics. A median of 72% challenge participants took part in the survey. According to our results, knowledge exchange was the primary incentive (70%) for participation, while the reception of prize money played only a minor role (16%). While a median of 80 working hours was spent on method development, a large portion of participants stated that they did not have enough time for method development (32%). 25% perceived the infrastructure to be a bottleneck. Overall, 94% of all solutions were deep learning-based. Of these, 84% were based on standard architectures. 43% of the respondents reported that the data samples (e.g., images) were too large to be processed at once. This was most commonly addressed by patch-based training (69%), downsampling (37%), and solving 3D analysis tasks as a series of 2D tasks. K-fold cross-validation on the training set was performed by only 37% of the participants and only 50% of the participants performed ensembling based on multiple identical models (61%) or heterogeneous models (39%). 48% of the respondents applied postprocessing steps.
CholecTriplet2021: A benchmark challenge for surgical action triplet recognition
Apr 10, 2022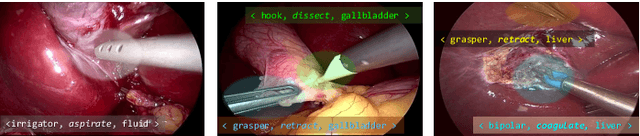
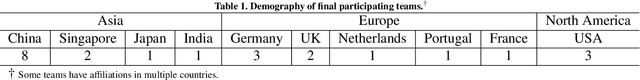
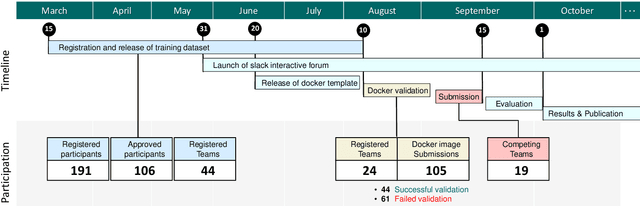
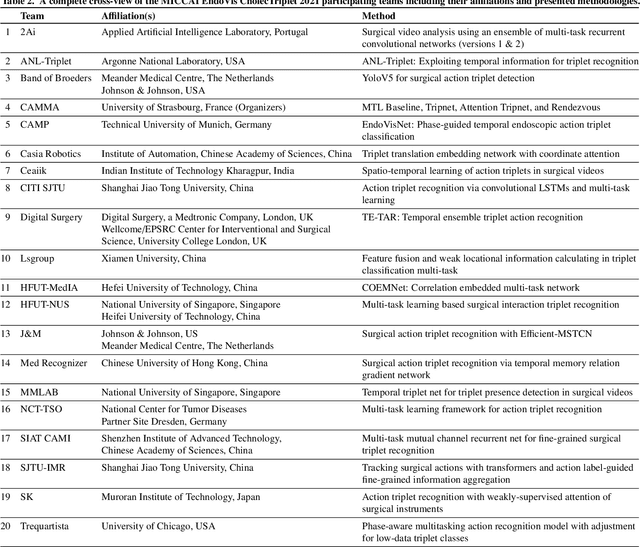
Abstract:Context-aware decision support in the operating room can foster surgical safety and efficiency by leveraging real-time feedback from surgical workflow analysis. Most existing works recognize surgical activities at a coarse-grained level, such as phases, steps or events, leaving out fine-grained interaction details about the surgical activity; yet those are needed for more helpful AI assistance in the operating room. Recognizing surgical actions as triplets of <instrument, verb, target> combination delivers comprehensive details about the activities taking place in surgical videos. This paper presents CholecTriplet2021: an endoscopic vision challenge organized at MICCAI 2021 for the recognition of surgical action triplets in laparoscopic videos. The challenge granted private access to the large-scale CholecT50 dataset, which is annotated with action triplet information. In this paper, we present the challenge setup and assessment of the state-of-the-art deep learning methods proposed by the participants during the challenge. A total of 4 baseline methods from the challenge organizers and 19 new deep learning algorithms by competing teams are presented to recognize surgical action triplets directly from surgical videos, achieving mean average precision (mAP) ranging from 4.2% to 38.1%. This study also analyzes the significance of the results obtained by the presented approaches, performs a thorough methodological comparison between them, in-depth result analysis, and proposes a novel ensemble method for enhanced recognition. Our analysis shows that surgical workflow analysis is not yet solved, and also highlights interesting directions for future research on fine-grained surgical activity recognition which is of utmost importance for the development of AI in surgery.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge