Ankur Kapoor
Guidance for Intra-cardiac Echocardiography Manipulation to Maintain Continuous Therapy Device Tip Visibility
May 08, 2025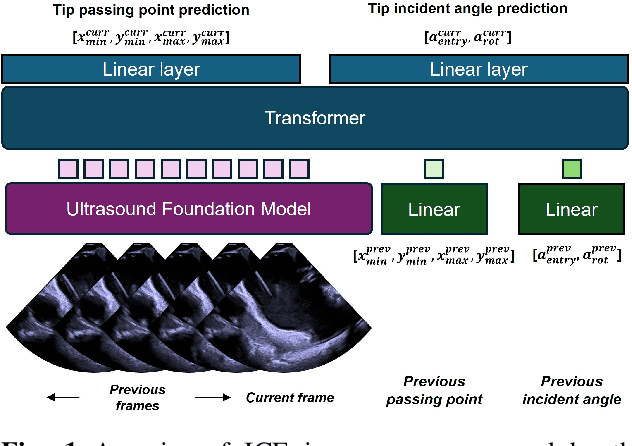
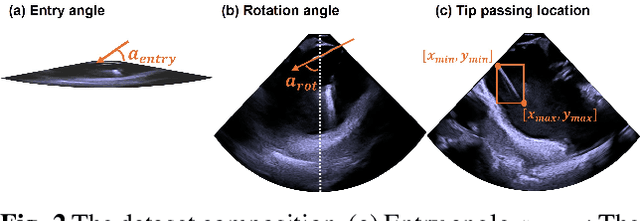
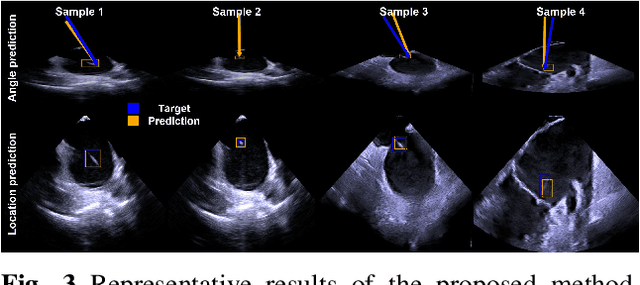
Abstract:Intra-cardiac Echocardiography (ICE) plays a critical role in Electrophysiology (EP) and Structural Heart Disease (SHD) interventions by providing real-time visualization of intracardiac structures. However, maintaining continuous visibility of the therapy device tip remains a challenge due to frequent adjustments required during manual ICE catheter manipulation. To address this, we propose an AI-driven tracking model that estimates the device tip incident angle and passing point within the ICE imaging plane, ensuring continuous visibility and facilitating robotic ICE catheter control. A key innovation of our approach is the hybrid dataset generation strategy, which combines clinical ICE sequences with synthetic data augmentation to enhance model robustness. We collected ICE images in a water chamber setup, equipping both the ICE catheter and device tip with electromagnetic (EM) sensors to establish precise ground-truth locations. Synthetic sequences were created by overlaying catheter tips onto real ICE images, preserving motion continuity while simulating diverse anatomical scenarios. The final dataset consists of 5,698 ICE-tip image pairs, ensuring comprehensive training coverage. Our model architecture integrates a pretrained ultrasound (US) foundation model, trained on 37.4M echocardiography images, for feature extraction. A transformer-based network processes sequential ICE frames, leveraging historical passing points and incident angles to improve prediction accuracy. Experimental results demonstrate that our method achieves 3.32 degree entry angle error, 12.76 degree rotation angle error. This AI-driven framework lays the foundation for real-time robotic ICE catheter adjustments, minimizing operator workload while ensuring consistent therapy device visibility. Future work will focus on expanding clinical datasets to further enhance model generalization.
Pose Estimation for Intra-cardiac Echocardiography Catheter via AI-Based Anatomical Understanding
May 07, 2025



Abstract:Intra-cardiac Echocardiography (ICE) plays a crucial role in Electrophysiology (EP) and Structural Heart Disease (SHD) interventions by providing high-resolution, real-time imaging of cardiac structures. However, existing navigation methods rely on electromagnetic (EM) tracking, which is susceptible to interference and position drift, or require manual adjustments based on operator expertise. To overcome these limitations, we propose a novel anatomy-aware pose estimation system that determines the ICE catheter position and orientation solely from ICE images, eliminating the need for external tracking sensors. Our approach leverages a Vision Transformer (ViT)-based deep learning model, which captures spatial relationships between ICE images and anatomical structures. The model is trained on a clinically acquired dataset of 851 subjects, including ICE images paired with position and orientation labels normalized to the left atrium (LA) mesh. ICE images are patchified into 16x16 embeddings and processed through a transformer network, where a [CLS] token independently predicts position and orientation via separate linear layers. The model is optimized using a Mean Squared Error (MSE) loss function, balancing positional and orientational accuracy. Experimental results demonstrate an average positional error of 9.48 mm and orientation errors of (16.13 deg, 8.98 deg, 10.47 deg) across x, y, and z axes, confirming the model accuracy. Qualitative assessments further validate alignment between predicted and target views within 3D cardiac meshes. This AI-driven system enhances procedural efficiency, reduces operator workload, and enables real-time ICE catheter localization for tracking-free procedures. The proposed method can function independently or complement existing mapping systems like CARTO, offering a transformative approach to ICE-guided interventions.
Multi-Agent Reinforcement Learning with Long-Term Performance Objectives for Service Workforce Optimization
Mar 03, 2025



Abstract:Workforce optimization plays a crucial role in efficient organizational operations where decision-making may span several different administrative and time scales. For instance, dispatching personnel to immediate service requests while managing talent acquisition with various expertise sets up a highly dynamic optimization problem. Existing work focuses on specific sub-problems such as resource allocation and facility location, which are solved with heuristics like local-search and, more recently, deep reinforcement learning. However, these may not accurately represent real-world scenarios where such sub-problems are not fully independent. Our aim is to fill this gap by creating a simulator that models a unified workforce optimization problem. Specifically, we designed a modular simulator to support the development of reinforcement learning methods for integrated workforce optimization problems. We focus on three interdependent aspects: personnel dispatch, workforce management, and personnel positioning. The simulator provides configurable parameterizations to help explore dynamic scenarios with varying levels of stochasticity and non-stationarity. To facilitate benchmarking and ablation studies, we also include heuristic and RL baselines for the above mentioned aspects.
AI-driven View Guidance System in Intra-cardiac Echocardiography Imaging
Sep 26, 2024



Abstract:Intra-cardiac Echocardiography (ICE) is a crucial imaging modality used in electrophysiology (EP) and structural heart disease (SHD) interventions, providing real-time, high-resolution views from within the heart. Despite its advantages, effective manipulation of the ICE catheter requires significant expertise, which can lead to inconsistent outcomes, particularly among less experienced operators. To address this challenge, we propose an AI-driven closed-loop view guidance system with human-in-the-loop feedback, designed to assist users in navigating ICE imaging without requiring specialized knowledge. Our method models the relative position and orientation vectors between arbitrary views and clinically defined ICE views in a spatial coordinate system, guiding users on how to manipulate the ICE catheter to transition from the current view to the desired view over time. Operating in a closed-loop configuration, the system continuously predicts and updates the necessary catheter manipulations, ensuring seamless integration into existing clinical workflows. The effectiveness of the proposed system is demonstrated through a simulation-based evaluation, achieving an 89% success rate with the 6532 test dataset, highlighting its potential to improve the accuracy and efficiency of ICE imaging procedures.
Automated CT Lung Cancer Screening Workflow using 3D Camera
Sep 27, 2023Abstract:Despite recent developments in CT planning that enabled automation in patient positioning, time-consuming scout scans are still needed to compute dose profile and ensure the patient is properly positioned. In this paper, we present a novel method which eliminates the need for scout scans in CT lung cancer screening by estimating patient scan range, isocenter, and Water Equivalent Diameter (WED) from 3D camera images. We achieve this task by training an implicit generative model on over 60,000 CT scans and introduce a novel approach for updating the prediction using real-time scan data. We demonstrate the effectiveness of our method on a testing set of 110 pairs of depth data and CT scan, resulting in an average error of 5mm in estimating the isocenter, 13mm in determining the scan range, 10mm and 16mm in estimating the AP and lateral WED respectively. The relative WED error of our method is 4%, which is well within the International Electrotechnical Commission (IEC) acceptance criteria of 10%.
AI-based Agents for Automated Robotic Endovascular Guidewire Manipulation
Apr 18, 2023Abstract:Endovascular guidewire manipulation is essential for minimally-invasive clinical applications (Percutaneous Coronary Intervention (PCI), Mechanical thrombectomy techniques for acute ischemic stroke (AIS), or Transjugular intrahepatic portosystemic shunt (TIPS)). All procedures commonly require 3D vessel geometries from 3D CTA (Computed Tomography Angiography) images. During these procedures, the clinician generally places a guiding catheter in the ostium of the relevant vessel and then manipulates a wire through the catheter and across the blockage. The clinician only uses X-ray fluoroscopy intermittently to visualize and guide the catheter, guidewire, and other devices. However, clinicians still passively control guidewires/catheters by relying on limited indirect observation (i.e., 2D partial view of devices, and intermittent updates due to radiation limit) from X-ray fluoroscopy. Modeling and controlling the guidewire manipulation in coronary vessels remains challenging because of the complicated interaction between guidewire motions with different physical properties (i.e., loads, coating) and vessel geometries with lumen conditions resulting in a highly non-linear system. This paper introduces a scalable learning pipeline to train AI-based agent models toward automated endovascular predictive device controls. First, we create a scalable environment by pre-processing 3D CTA images, providing patient-specific 3D vessel geometry and the centerline of the coronary. Next, we apply a large quantity of randomly generated motion sequences from the proximal end to generate wire states associated with each environment using a physics-based device simulator. Then, we reformulate the control problem to a sequence-to-sequence learning problem, in which we use a Transformer-based model, trained to handle non-linear sequential forward/inverse transition functions.
Automated Catheter Tip Repositioning for Intra-cardiac Echocardiography
Jan 21, 2022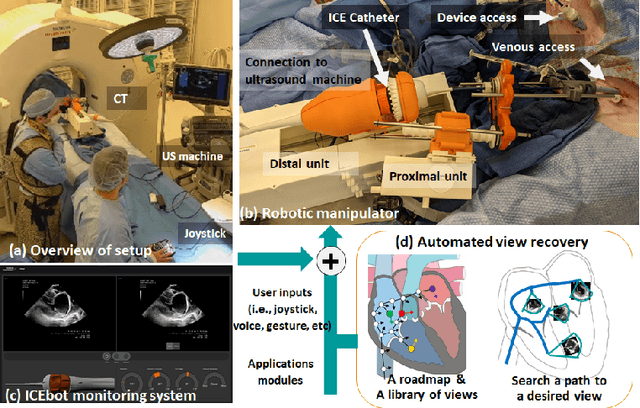

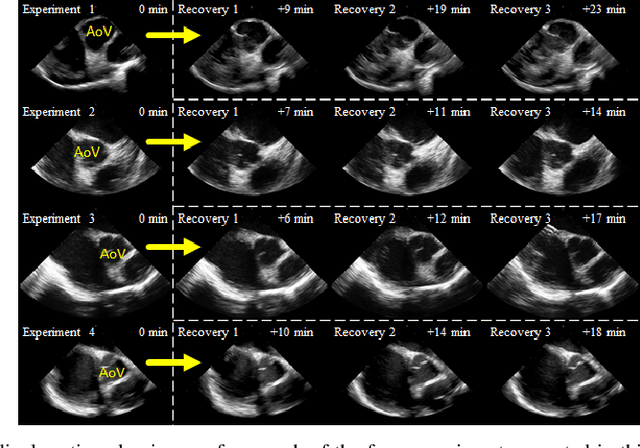
Abstract:Purpose: Intra-Cardiac Echocardiography (ICE) is a powerful imaging modality for guiding cardiac electrophysiology and structural heart interventions. ICE provides real-time observation of anatomy and devices, while enabling direct monitoring of potential complications. In single operator settings, the physician needs to switch back-and-forth between the ICE catheter and therapy device, making continuous ICE support impossible. Two operators setup are therefore sometimes implemented, with the challenge of increase room occupation and cost. Two operator setups are sometimes implemented, but increase procedural costs and room occupation. Methods: ICE catheter robotic control system is developed with automated catheter tip repositioning (i.e. view recovery) method, which can reproduce important views previously navigated to and saved by the user. The performance of the proposed method is demonstrated and evaluated in a combination of heart phantom and animal experiments. Results: Automated ICE view recovery achieved catheter tip position accuracy of 2.09 +/-0.90 mm and catheter image orientation accuracy of 3.93 +/- 2.07 degree in animal studies, and 0.67 +/- 0.79 mm and 0.37 +/- 0.19 degree in heart phantom studies, respectively. Our proposed method is also successfully used during transeptal puncture in animals without complications, showing the possibility for fluoro-less transeptal puncture with ICE catheter robot. Conclusion: Robotic ICE imaging has the potential to provide precise and reproducible anatomical views, which can reduce overall execution time, labor burden of procedures, and x-ray usage for a range of cardiac procedures. Keywords: Automated View Recovery, Path Planning, Intra-cardiac echocardiography (ICE), Catheter, Tendon-driven manipulator, Cardiac Imaging
A Wide-area, Low-latency, and Power-efficient 6-DoF Pose Tracking System for Rigid Objects
Sep 15, 2021



Abstract:Position sensitive detectors (PSDs) offer possibility to track single active marker's two (or three) degrees of freedom (DoF) position with a high accuracy, while having a fast response time with high update frequency and low latency, all using a very simple signal processing circuit. However they are not particularly suitable for 6-DoF object pose tracking system due to lack of orientation measurement, limited tracking range, and sensitivity to environmental variation. We propose a novel 6-DoF pose tracking system for a rigid object tracking requiring a single active marker. The proposed system uses a stereo-based PSD pair and multiple Inertial Measurement Units (IMUs). This is done based on a practical approach to identify and control the power of Infrared-Light Emitting Diode (IR-LED) active markers, with an aim to increase the tracking work space and reduce the power consumption. Our proposed tracking system is validated with three different work space sizes and for static and dynamic positional accuracy using robotic arm manipulator with three different dynamic motion patterns. The results show that the static position root-mean-square (RMS) error is 0.6mm. The dynamic position RMS error is 0.7-0.9mm. The orientation RMS error is between 0.04 and 0.9 degree at varied dynamic motion. Overall, our proposed tracking system is capable of tracking a rigid object pose with sub-millimeter accuracy at the mid range of the work space and sub-degree accuracy for all work space under a lab setting.
Non-linear Hysteresis Compensation of a Tendon-sheath-driven Robotic Manipulator using Motor Current
Nov 03, 2020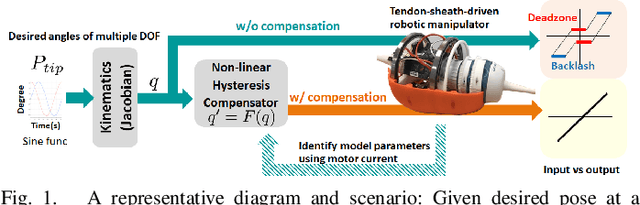

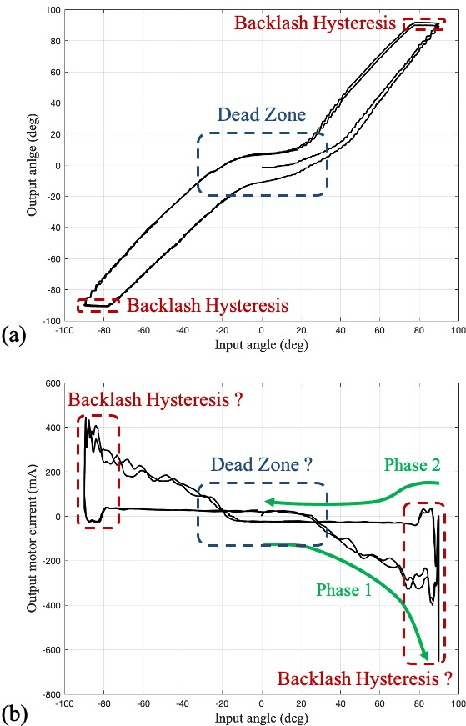
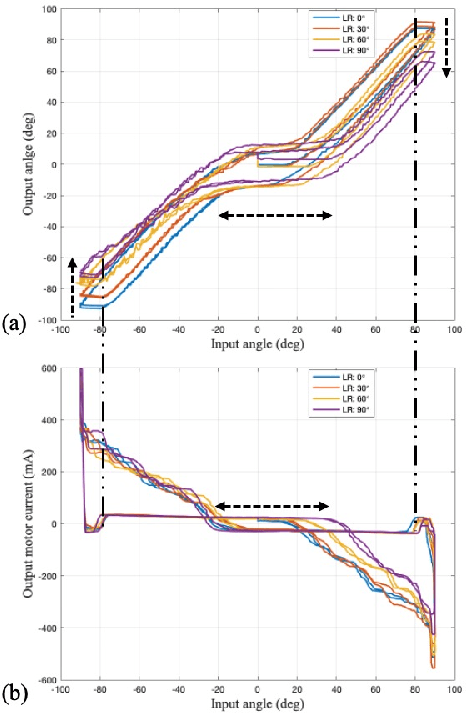
Abstract:Tendon-sheath-driven manipulators (TSM) are widely used in minimally invasive surgical systems due to their long, thin shape, flexibility, and compliance making them easily steerable in narrow or tortuous environments. Many commercial TSM-based medical devices have non-linear phenomena resulting from their composition such as backlash and dead zone hysteresis, which lead to a considerable challenge for achieving precise control of the end effector pose. However, many recent works in the literature do not consider the combined effects and compensation of these phenomena, and less focus on practical ways to identify model parameters in real field. In this paper, we propose a simplified piece-wise linear model to compensate both backlash and dead zone hysteresis together. Further, we introduce a practical method to identify model parameters using motor current from a robotic controller for the TSM. We analyze our proposed methods with multiple Intra-cardiac Echocardiography catheters, which are typical commercial example of TSM. Our results show that the errors from backlash and dead zone hysteresis are considerably reduced and therefore the accuracy of robotic control is improved when applying the presented methods.
Towards Automatic Manipulation of Intra-cardiac Echocardiography Catheter
Sep 12, 2020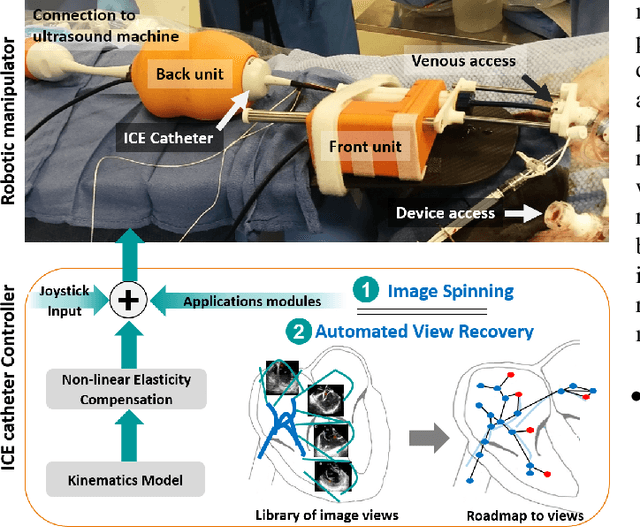
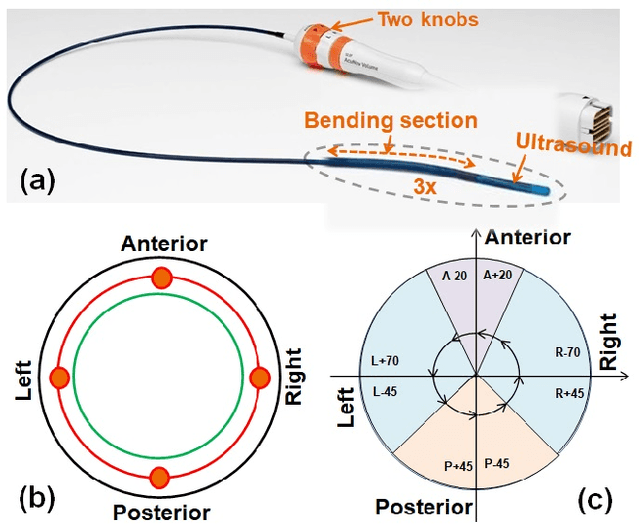
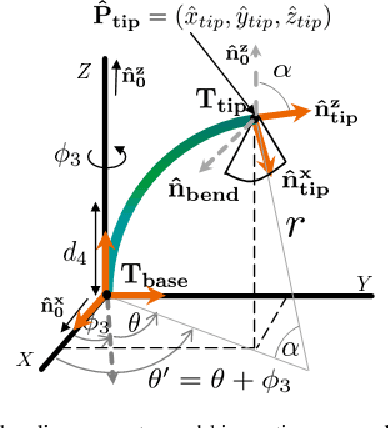
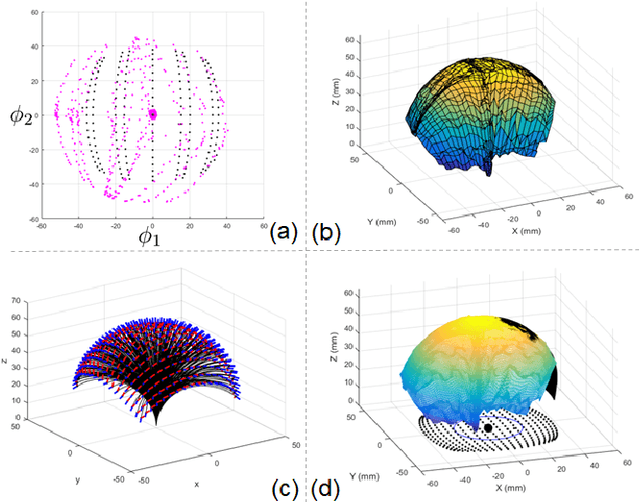
Abstract:Intra-cardiac Echocardiography (ICE) has been evolving as a real-time imaging modality of choice for guiding electrophiosology and structural heart interventions. ICE provides real-time imaging of anatomy, catheters, and complications such as pericardial effusion or thrombus formation. However, there now exists a high cognitive demand on physicians with the increased reliance on intraprocedural imaging. In response, we present a robotic manipulator for AcuNav ICE catheters to alleviate the physician's burden and support applied methods for more automated. Herein, we introduce two methods towards these goals: (1) a data-driven method to compensate kinematic model errors due to non-linear elasticity in catheter bending, providing more precise robotic control and (2) an automated image recovery process that allows physicians to bookmark images during intervention and automatically return with the push of a button. To validate our error compensation method, we demonstrate a complex rotation of the ultrasound imaging plane evaluated on benchtop. Automated view recovery is validated by repeated imaging of landmarks on benchtop and in vivo experiments with position- and image-based analysis. Results support that a robotic-assist system for more autonomous ICE can provide a safe and efficient tool, potentially reducing the execution time and allowing more complex procedures to become common place.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge