Èric Lluch
AI-based Agents for Automated Robotic Endovascular Guidewire Manipulation
Apr 18, 2023Abstract:Endovascular guidewire manipulation is essential for minimally-invasive clinical applications (Percutaneous Coronary Intervention (PCI), Mechanical thrombectomy techniques for acute ischemic stroke (AIS), or Transjugular intrahepatic portosystemic shunt (TIPS)). All procedures commonly require 3D vessel geometries from 3D CTA (Computed Tomography Angiography) images. During these procedures, the clinician generally places a guiding catheter in the ostium of the relevant vessel and then manipulates a wire through the catheter and across the blockage. The clinician only uses X-ray fluoroscopy intermittently to visualize and guide the catheter, guidewire, and other devices. However, clinicians still passively control guidewires/catheters by relying on limited indirect observation (i.e., 2D partial view of devices, and intermittent updates due to radiation limit) from X-ray fluoroscopy. Modeling and controlling the guidewire manipulation in coronary vessels remains challenging because of the complicated interaction between guidewire motions with different physical properties (i.e., loads, coating) and vessel geometries with lumen conditions resulting in a highly non-linear system. This paper introduces a scalable learning pipeline to train AI-based agent models toward automated endovascular predictive device controls. First, we create a scalable environment by pre-processing 3D CTA images, providing patient-specific 3D vessel geometry and the centerline of the coronary. Next, we apply a large quantity of randomly generated motion sequences from the proximal end to generate wire states associated with each environment using a physics-based device simulator. Then, we reformulate the control problem to a sequence-to-sequence learning problem, in which we use a Transformer-based model, trained to handle non-linear sequential forward/inverse transition functions.
Graph convolutional regression of cardiac depolarization from sparse endocardial maps
Sep 28, 2020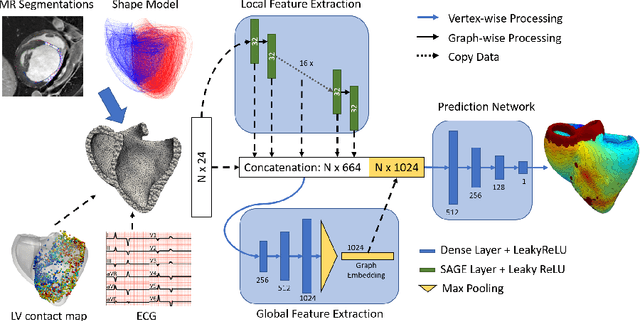

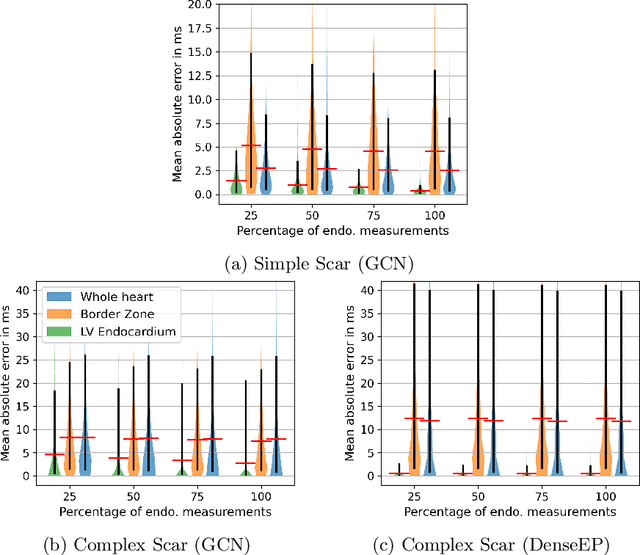
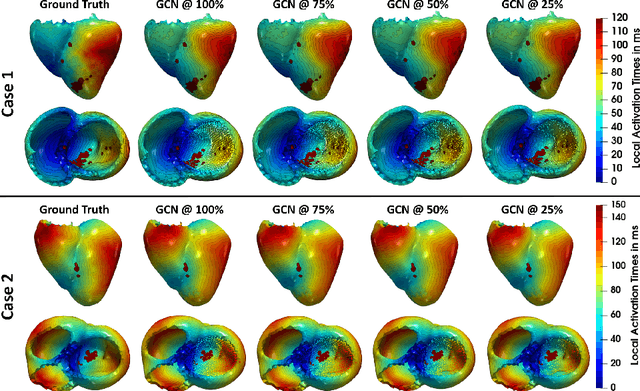
Abstract:Electroanatomic mapping as routinely acquired in ablation therapy of ventricular tachycardia is the gold standard method to identify the arrhythmogenic substrate. To reduce the acquisition time and still provide maps with high spatial resolution, we propose a novel deep learning method based on graph convolutional neural networks to estimate the depolarization time in the myocardium, given sparse catheter data on the left ventricular endocardium, ECG, and magnetic resonance images. The training set consists of data produced by a computational model of cardiac electrophysiology on a large cohort of synthetically generated geometries of ischemic hearts. The predicted depolarization pattern has good agreement with activation times computed by the cardiac electrophysiology model in a validation set of five swine heart geometries with complex scar and border zone morphologies. The mean absolute error hereby measures 8 ms on the entire myocardium when providing 50\% of the endocardial ground truth in over 500 computed depolarization patterns. Furthermore, when considering a complete animal data set with high density electroanatomic mapping data as reference, the neural network can accurately reproduce the endocardial depolarization pattern, even when a small percentage of measurements are provided as input features (mean absolute error of 7 ms with 50\% of input samples). The results show that the proposed method, trained on synthetically generated data, may generalize to real data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge