Tiziano Passerini
EchoVLM: Measurement-Grounded Multimodal Learning for Echocardiography
Dec 13, 2025Abstract:Echocardiography is the most widely used imaging modality in cardiology, yet its interpretation remains labor-intensive and inherently multimodal, requiring view recognition, quantitative measurements, qualitative assessments, and guideline-based reasoning. While recent vision-language models (VLMs) have achieved broad success in natural images and certain medical domains, their potential in echocardiography has been limited by the lack of large-scale, clinically grounded image-text datasets and the absence of measurement-based reasoning central to echo interpretation. We introduce EchoGround-MIMIC, the first measurement-grounded multimodal echocardiography dataset, comprising 19,065 image-text pairs from 1,572 patients with standardized views, structured measurements, measurement-grounded captions, and guideline-derived disease labels. Building on this resource, we propose EchoVLM, a vision-language model that incorporates two novel pretraining objectives: (i) a view-informed contrastive loss that encodes the view-dependent structure of echocardiographic imaging, and (ii) a negation-aware contrastive loss that distinguishes clinically critical negative from positive findings. Across five types of clinical applications with 36 tasks spanning multimodal disease classification, image-text retrieval, view classification, chamber segmentation, and landmark detection, EchoVLM achieves state-of-the-art performance (86.5% AUC in zero-shot disease classification and 95.1% accuracy in view classification). We demonstrate that clinically grounded multimodal pretraining yields transferable visual representations and establish EchoVLM as a foundation model for end-to-end echocardiography interpretation. We will release EchoGround-MIMIC and the data curation code, enabling reproducibility and further research in multimodal echocardiography interpretation.
ConceptVAE: Self-Supervised Fine-Grained Concept Disentanglement from 2D Echocardiographies
Feb 03, 2025



Abstract:While traditional self-supervised learning methods improve performance and robustness across various medical tasks, they rely on single-vector embeddings that may not capture fine-grained concepts such as anatomical structures or organs. The ability to identify such concepts and their characteristics without supervision has the potential to improve pre-training methods, and enable novel applications such as fine-grained image retrieval and concept-based outlier detection. In this paper, we introduce ConceptVAE, a novel pre-training framework that detects and disentangles fine-grained concepts from their style characteristics in a self-supervised manner. We present a suite of loss terms and model architecture primitives designed to discretise input data into a preset number of concepts along with their local style. We validate ConceptVAE both qualitatively and quantitatively, demonstrating its ability to detect fine-grained anatomical structures such as blood pools and septum walls from 2D cardiac echocardiographies. Quantitatively, ConceptVAE outperforms traditional self-supervised methods in tasks such as region-based instance retrieval, semantic segmentation, out-of-distribution detection, and object detection. Additionally, we explore the generation of in-distribution synthetic data that maintains the same concepts as the training data but with distinct styles, highlighting its potential for more calibrated data generation. Overall, our study introduces and validates a promising new pre-training technique based on concept-style disentanglement, opening multiple avenues for developing models for medical image analysis that are more interpretable and explainable than black-box approaches.
EchoApex: A General-Purpose Vision Foundation Model for Echocardiography
Oct 14, 2024



Abstract:Quantitative evaluation of echocardiography is essential for precise assessment of cardiac condition, monitoring disease progression, and guiding treatment decisions. The diverse nature of echo images, including variations in probe types, manufacturers, and pathologies, poses challenges for developing artificial intelligent models that can generalize across different clinical practice. We introduce EchoApex, the first general-purpose vision foundation model echocardiography with applications on a variety of clinical practice. Leveraging self-supervised learning, EchoApex is pretrained on over 20 million echo images from 11 clinical centres. By incorporating task-specific decoders and adapter modules, we demonstrate the effectiveness of EchoApex on 4 different kind of clinical applications with 28 sub-tasks, including view classification, interactive structure segmentation, left ventricle hypertrophy detection and automated ejection fraction estimation from view sequences. Compared to state-of-the-art task-specific models, EchoApex attains improved performance with a unified image encoding architecture, demonstrating the benefits of model pretraining at scale with in-domain data. Furthermore, EchoApex illustrates the potential for developing a general-purpose vision foundation model tailored specifically for echocardiography, capable of addressing a diverse range of clinical applications with high efficiency and efficacy.
Graph convolutional regression of cardiac depolarization from sparse endocardial maps
Sep 28, 2020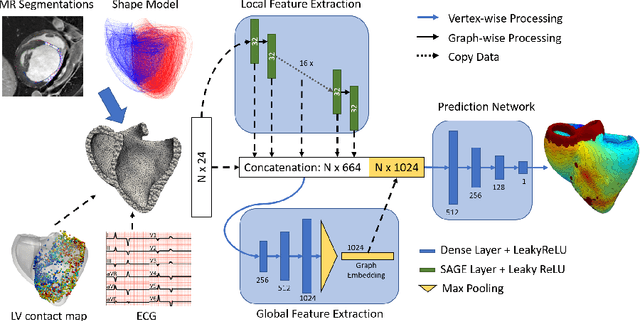

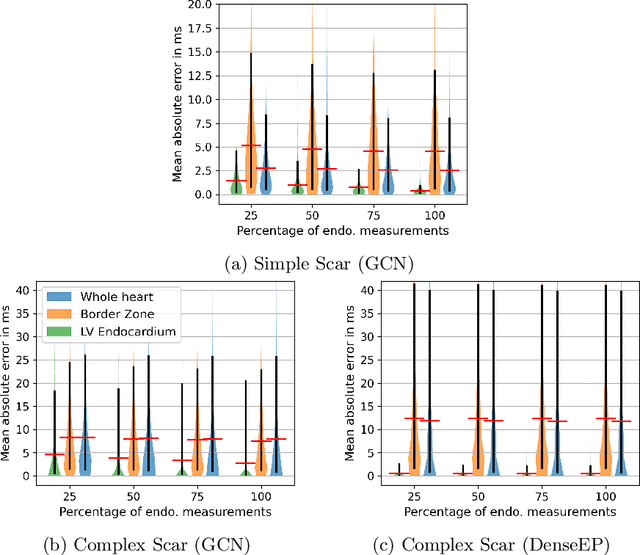
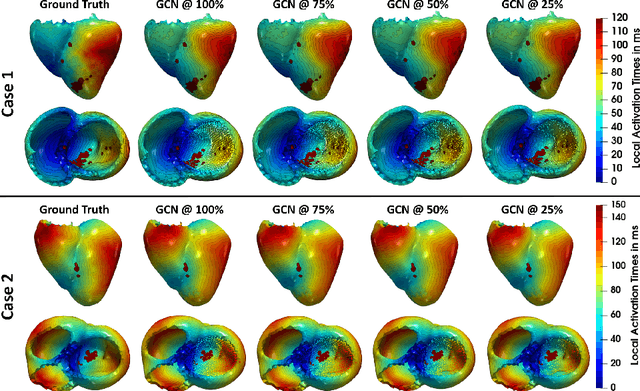
Abstract:Electroanatomic mapping as routinely acquired in ablation therapy of ventricular tachycardia is the gold standard method to identify the arrhythmogenic substrate. To reduce the acquisition time and still provide maps with high spatial resolution, we propose a novel deep learning method based on graph convolutional neural networks to estimate the depolarization time in the myocardium, given sparse catheter data on the left ventricular endocardium, ECG, and magnetic resonance images. The training set consists of data produced by a computational model of cardiac electrophysiology on a large cohort of synthetically generated geometries of ischemic hearts. The predicted depolarization pattern has good agreement with activation times computed by the cardiac electrophysiology model in a validation set of five swine heart geometries with complex scar and border zone morphologies. The mean absolute error hereby measures 8 ms on the entire myocardium when providing 50\% of the endocardial ground truth in over 500 computed depolarization patterns. Furthermore, when considering a complete animal data set with high density electroanatomic mapping data as reference, the neural network can accurately reproduce the endocardial depolarization pattern, even when a small percentage of measurements are provided as input features (mean absolute error of 7 ms with 50\% of input samples). The results show that the proposed method, trained on synthetically generated data, may generalize to real data.
Deep Neural Networks for ECG-free Cardiac Phase and End-Diastolic Frame Detection on Coronary Angiographies
Nov 07, 2018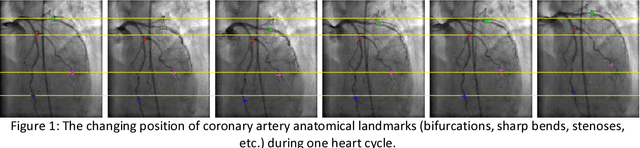
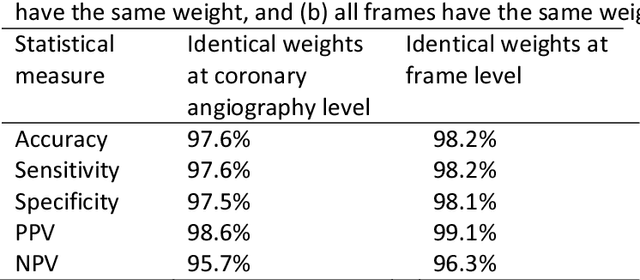
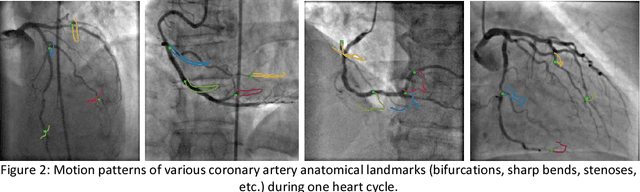
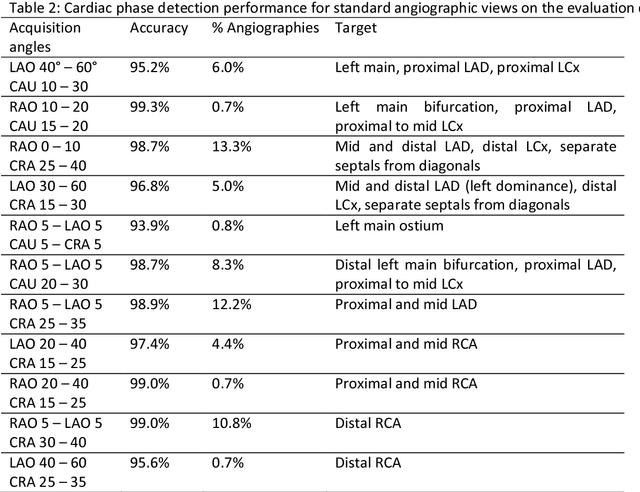
Abstract:Invasive coronary angiography (ICA) is the gold standard in Coronary Artery Disease (CAD) imaging. Detection of the end-diastolic frame (EDF) and, in general, cardiac phase detection on each temporal frame of a coronary angiography acquisition is of significant importance for the anatomical and non-invasive functional assessment of CAD. This task is generally performed via manual frame selection or semi-automated selection based on simultaneously acquired ECG signals - thus introducing the requirement of simultaneous ECG recordings. We evaluate the performance of a purely image based workflow based on deep neural networks for fully automated cardiac phase and EDF detection on coronary angiographies. A first deep neural network (DNN), trained to detect coronary arteries, is employed to preselect a subset of frames in which coronary arteries are well visible. A second DNN predicts cardiac phase labels for each frame. Only in the training and evaluation phases for the second DNN, ECG signals are used to provide ground truth labels for each angiographic frame. The networks were trained on 17800 coronary angiographies from 3900 patients and evaluated on 27900 coronary angiographies from 6250 patients. No exclusion criteria related to patient state, previous interventions, or pathology were formulated. Cardiac phase detection had an accuracy of 97.6%, a sensitivity of 97.6% and a specificity of 97.5% on the evaluation set. EDF prediction had a precision of 97.4% and a recall of 96.9%. Several sub-group analyses were performed, indicating that the cardiac phase detection performance is largely independent from acquisition angles and the heart rate of the patient. The average execution time of cardiac phase detection for one angiographic series was on average less than five seconds on a standard workstation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge