Lucian Itu
Anatomically aware dual-hop learning for pulmonary embolism detection in CT pulmonary angiograms
Mar 30, 2023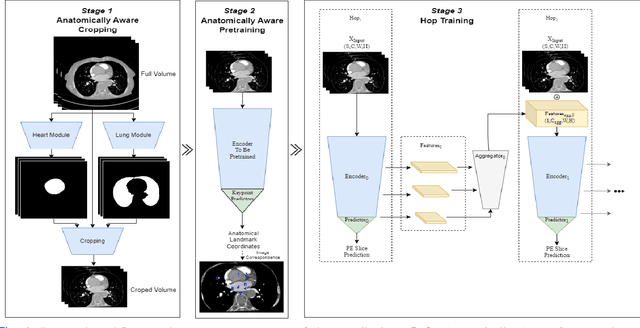
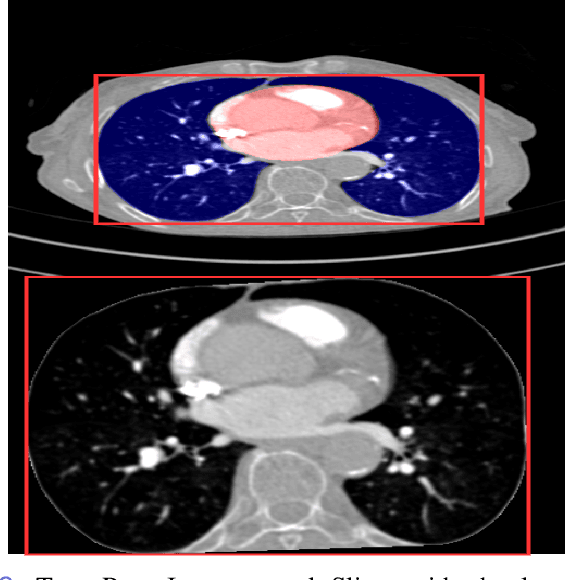
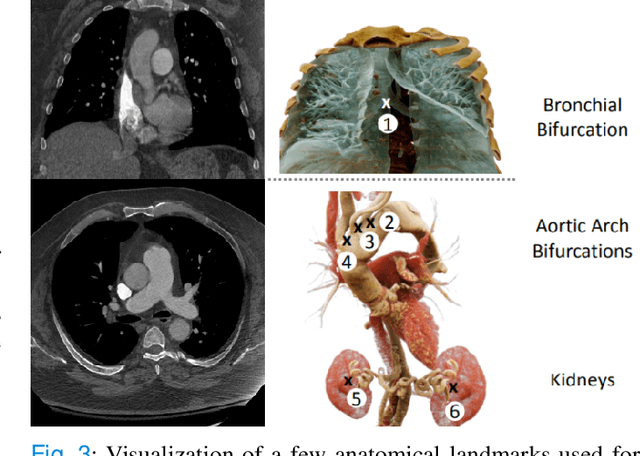
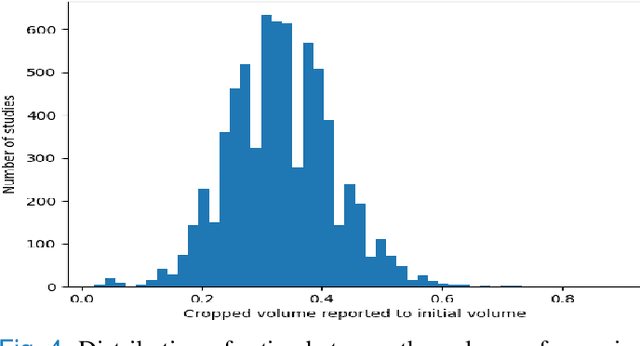
Abstract:Pulmonary Embolisms (PE) represent a leading cause of cardiovascular death. While medical imaging, through computed tomographic pulmonary angiography (CTPA), represents the gold standard for PE diagnosis, it is still susceptible to misdiagnosis or significant diagnosis delays, which may be fatal for critical cases. Despite the recently demonstrated power of deep learning to bring a significant boost in performance in a wide range of medical imaging tasks, there are still very few published researches on automatic pulmonary embolism detection. Herein we introduce a deep learning based approach, which efficiently combines computer vision and deep neural networks for pulmonary embolism detection in CTPA. Our method features novel improvements along three orthogonal axes: 1) automatic detection of anatomical structures; 2) anatomical aware pretraining, and 3) a dual-hop deep neural net for PE detection. We obtain state-of-the-art results on the publicly available multicenter large-scale RSNA dataset.
Deep Neural Networks for ECG-free Cardiac Phase and End-Diastolic Frame Detection on Coronary Angiographies
Nov 07, 2018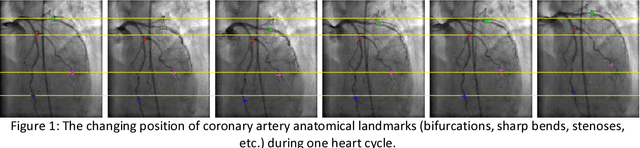
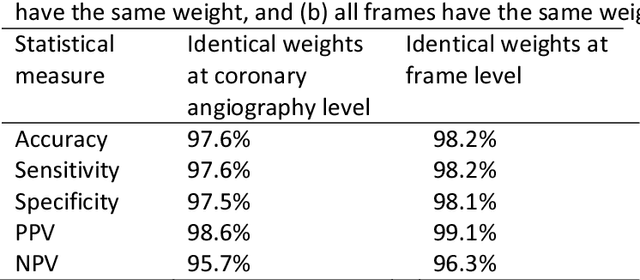
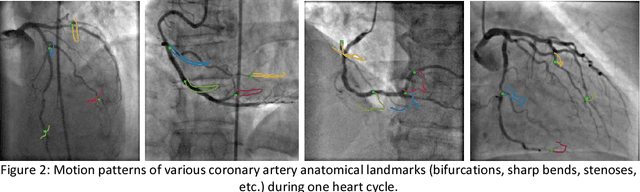
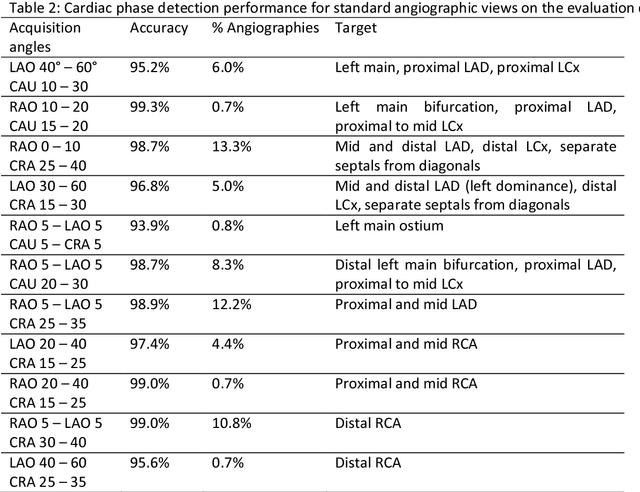
Abstract:Invasive coronary angiography (ICA) is the gold standard in Coronary Artery Disease (CAD) imaging. Detection of the end-diastolic frame (EDF) and, in general, cardiac phase detection on each temporal frame of a coronary angiography acquisition is of significant importance for the anatomical and non-invasive functional assessment of CAD. This task is generally performed via manual frame selection or semi-automated selection based on simultaneously acquired ECG signals - thus introducing the requirement of simultaneous ECG recordings. We evaluate the performance of a purely image based workflow based on deep neural networks for fully automated cardiac phase and EDF detection on coronary angiographies. A first deep neural network (DNN), trained to detect coronary arteries, is employed to preselect a subset of frames in which coronary arteries are well visible. A second DNN predicts cardiac phase labels for each frame. Only in the training and evaluation phases for the second DNN, ECG signals are used to provide ground truth labels for each angiographic frame. The networks were trained on 17800 coronary angiographies from 3900 patients and evaluated on 27900 coronary angiographies from 6250 patients. No exclusion criteria related to patient state, previous interventions, or pathology were formulated. Cardiac phase detection had an accuracy of 97.6%, a sensitivity of 97.6% and a specificity of 97.5% on the evaluation set. EDF prediction had a precision of 97.4% and a recall of 96.9%. Several sub-group analyses were performed, indicating that the cardiac phase detection performance is largely independent from acquisition angles and the heart rate of the patient. The average execution time of cardiac phase detection for one angiographic series was on average less than five seconds on a standard workstation.
A Self-Taught Artificial Agent for Multi-Physics Computational Model Personalization
May 01, 2016
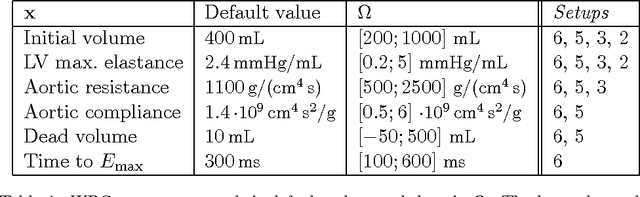
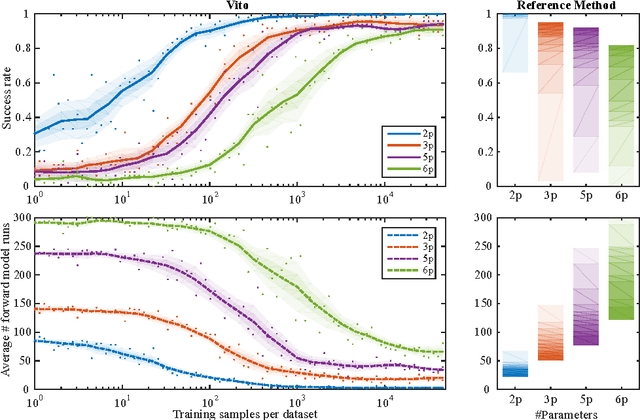
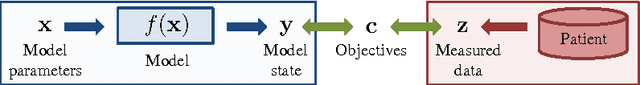
Abstract:Personalization is the process of fitting a model to patient data, a critical step towards application of multi-physics computational models in clinical practice. Designing robust personalization algorithms is often a tedious, time-consuming, model- and data-specific process. We propose to use artificial intelligence concepts to learn this task, inspired by how human experts manually perform it. The problem is reformulated in terms of reinforcement learning. In an off-line phase, Vito, our self-taught artificial agent, learns a representative decision process model through exploration of the computational model: it learns how the model behaves under change of parameters. The agent then automatically learns an optimal strategy for on-line personalization. The algorithm is model-independent; applying it to a new model requires only adjusting few hyper-parameters of the agent and defining the observations to match. The full knowledge of the model itself is not required. Vito was tested in a synthetic scenario, showing that it could learn how to optimize cost functions generically. Then Vito was applied to the inverse problem of cardiac electrophysiology and the personalization of a whole-body circulation model. The obtained results suggested that Vito could achieve equivalent, if not better goodness of fit than standard methods, while being more robust (up to 11% higher success rates) and with faster (up to seven times) convergence rate. Our artificial intelligence approach could thus make personalization algorithms generalizable and self-adaptable to any patient and any model.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge