Saikiran Rapaka
Label up: Learning Pulmonary Embolism Segmentation from Image Level Annotation through Model Explainability
Dec 10, 2024



Abstract:Pulmonary Embolisms (PE) are a leading cause of cardiovascular death. Computed tomographic pulmonary angiography (CTPA) stands as the gold standard for diagnosing pulmonary embolisms (PE) and there has been a lot of interest in developing AI-based models for assisting in PE diagnosis. Performance of these algorithms has been hindered by the scarcity of annotated data, especially those with fine-grained delineation of the thromboembolic burden. In this paper we attempt to address this issue by introducing a weakly supervised learning pipeline, that leverages model explainability to generate fine-grained (pixel level) masks for embolisms starting from more coarse-grained (binary, image level) PE annotations. Furthermore, we show that training models using the automatically generated pixel annotations yields good PE localization performance. We demonstrate the effectiveness of our pipeline on the large-scale, multi-center RSPECT augmented dataset for PE detection and localization.
AI-based, automated chamber volumetry from gated, non-contrast CT
Oct 25, 2023Abstract:Background: Accurate chamber volumetry from gated, non-contrast cardiac CT (NCCT) scans can be useful for potential screening of heart failure. Objectives: To validate a new, fully automated, AI-based method for cardiac volume and myocardial mass quantification from NCCT scans compared to contrasted CT Angiography (CCTA). Methods: Of a retrospectively collected cohort of 1051 consecutive patients, 420 patients had both NCCT and CCTA scans at mid-diastolic phase, excluding patients with cardiac devices. Ground truth values were obtained from the CCTA scans. Results: The NCCT volume computation shows good agreement with ground truth values. Volume differences [95% CI ] and correlation coefficients were: -9.6 [-45; 26] mL, r = 0.98 for LV Total, -5.4 [-24; 13] mL, r = 0.95 for LA, -8.7 [-45; 28] mL, r = 0.94 for RV, -5.2 [-27; 17] mL, r = 0.92 for RA, -3.2 [-42; 36] mL, r = 0.91 for LV blood pool, and -6.7 [-39; 26] g, r = 0.94 for LV wall mass, respectively. Mean relative volume errors of less than 7% were obtained for all chambers. Conclusions: Fully automated assessment of chamber volumes from NCCT scans is feasible and correlates well with volumes obtained from contrast study.
Anatomically aware dual-hop learning for pulmonary embolism detection in CT pulmonary angiograms
Mar 30, 2023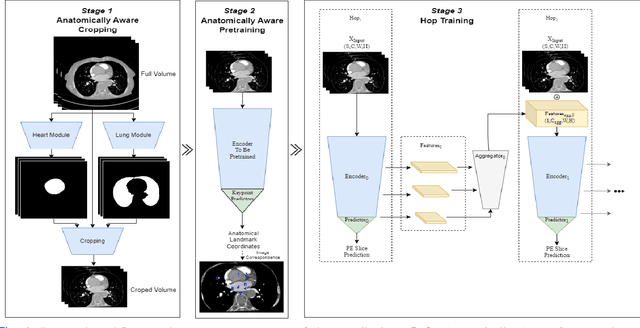
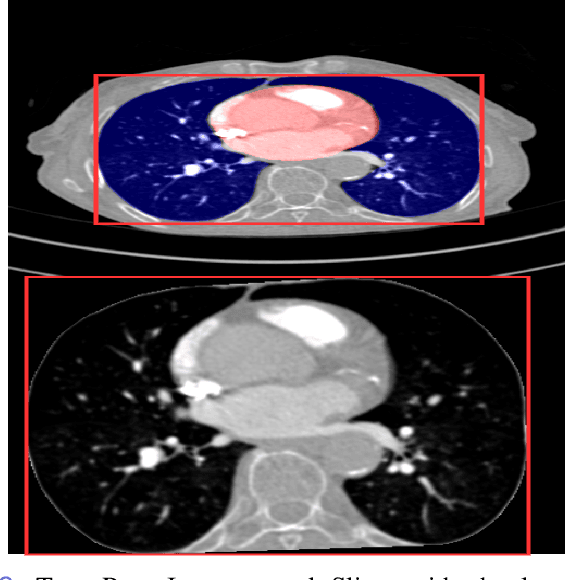
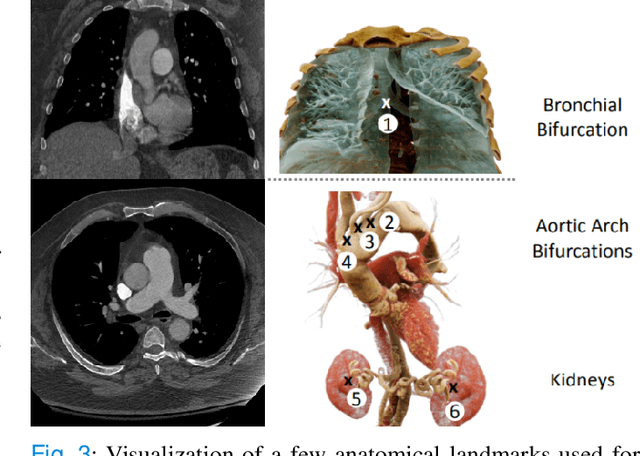
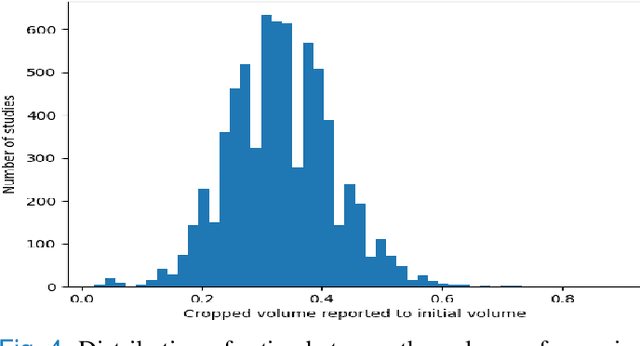
Abstract:Pulmonary Embolisms (PE) represent a leading cause of cardiovascular death. While medical imaging, through computed tomographic pulmonary angiography (CTPA), represents the gold standard for PE diagnosis, it is still susceptible to misdiagnosis or significant diagnosis delays, which may be fatal for critical cases. Despite the recently demonstrated power of deep learning to bring a significant boost in performance in a wide range of medical imaging tasks, there are still very few published researches on automatic pulmonary embolism detection. Herein we introduce a deep learning based approach, which efficiently combines computer vision and deep neural networks for pulmonary embolism detection in CTPA. Our method features novel improvements along three orthogonal axes: 1) automatic detection of anatomical structures; 2) anatomical aware pretraining, and 3) a dual-hop deep neural net for PE detection. We obtain state-of-the-art results on the publicly available multicenter large-scale RSNA dataset.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge