Jonathan Sperl
Anatomically aware dual-hop learning for pulmonary embolism detection in CT pulmonary angiograms
Mar 30, 2023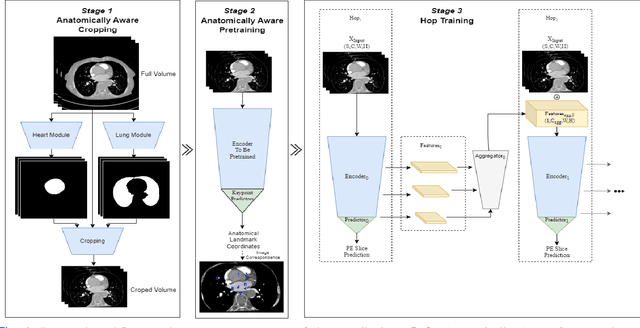
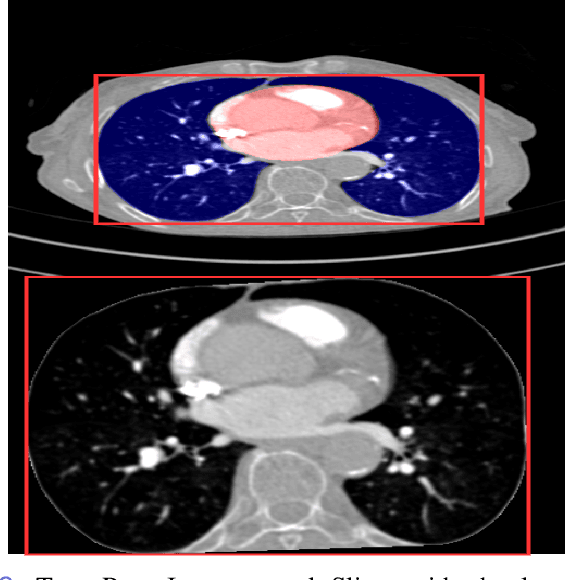
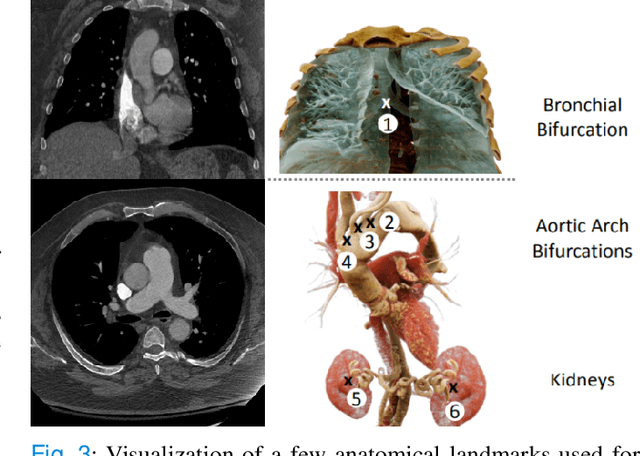
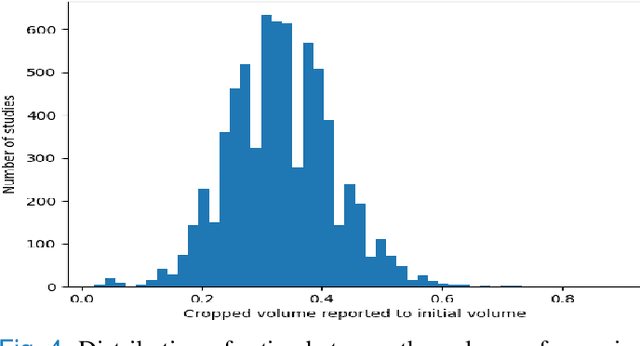
Abstract:Pulmonary Embolisms (PE) represent a leading cause of cardiovascular death. While medical imaging, through computed tomographic pulmonary angiography (CTPA), represents the gold standard for PE diagnosis, it is still susceptible to misdiagnosis or significant diagnosis delays, which may be fatal for critical cases. Despite the recently demonstrated power of deep learning to bring a significant boost in performance in a wide range of medical imaging tasks, there are still very few published researches on automatic pulmonary embolism detection. Herein we introduce a deep learning based approach, which efficiently combines computer vision and deep neural networks for pulmonary embolism detection in CTPA. Our method features novel improvements along three orthogonal axes: 1) automatic detection of anatomical structures; 2) anatomical aware pretraining, and 3) a dual-hop deep neural net for PE detection. We obtain state-of-the-art results on the publicly available multicenter large-scale RSNA dataset.
Accelerated Reconstruction of Perfusion-Weighted MRI Enforcing Jointly Local and Nonlocal Spatio-temporal Constraints
Aug 25, 2017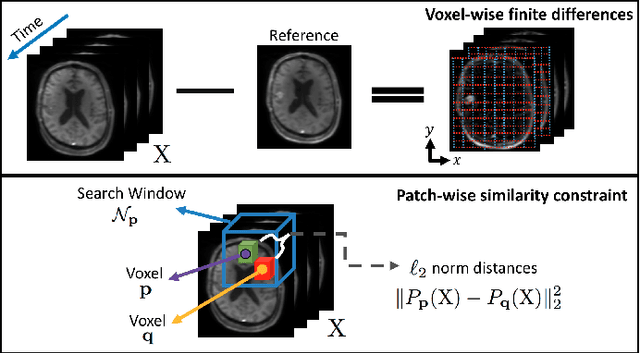
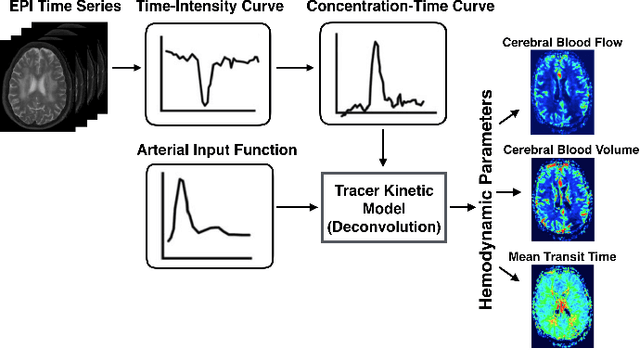
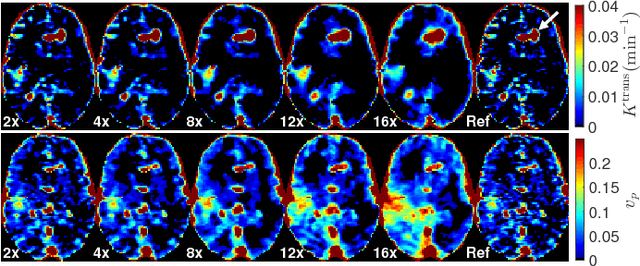
Abstract:Perfusion-weighted magnetic resonance imaging (MRI) is an imaging technique that allows one to measure tissue perfusion in an organ of interest through the injection of an intravascular paramagnetic contrast agent (CA). Due to a preference for high temporal and spatial resolution in many applications, this modality could significantly benefit from accelerated data acquisitions. In this paper, we specifically address the problem of reconstructing perfusion MR image series from a subset of k-space data. Our proposed approach is motivated by the observation that temporal variations (dynamics) in perfusion imaging often exhibit correlation across different spatial scales. Hence, we propose a model that jointly penalizes the voxel-wise deviations in temporal gradient images obtained based on a baseline, and the patch-wise dissimilarities between the spatio-temporal neighborhoods of entire image sequence. We validate our method on dynamic susceptibility contrast (DSC)-MRI and dynamic contrast-enhanced (DCE)-MRI brain perfusion datasets acquired from 10 tumor patients in total. We provide extensive analysis of reconstruction performance and perfusion parameter estimation in comparison to state-of-the-art reconstruction methods. Experimental results on clinical datasets demonstrate that our reconstruction model can potentially achieve up to 8-fold acceleration by enabling accurate estimation of perfusion parameters while preserving spatial image details and reconstructing the complete perfusion time-intensity curves (TICs).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge