Cagdas Ulas
Direct Estimation of Pharmacokinetic Parameters from DCE-MRI using Deep CNN with Forward Physical Model Loss
Jun 12, 2018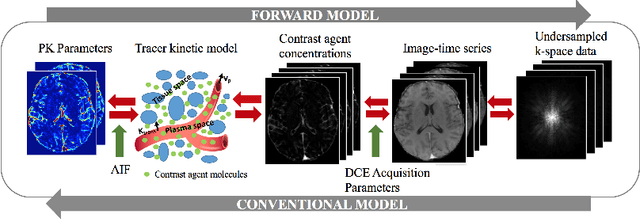
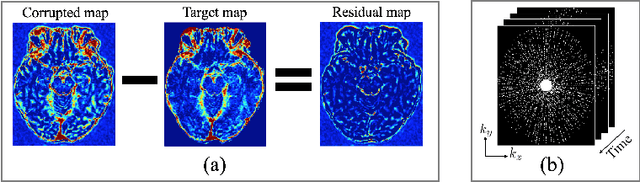
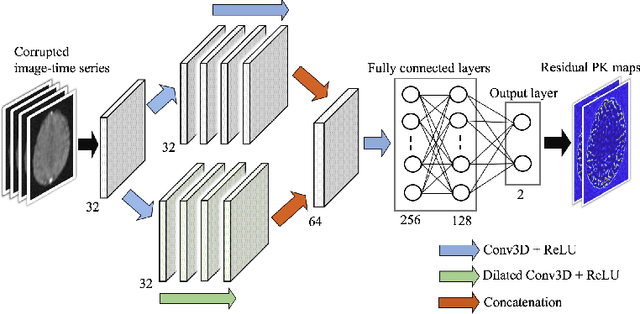
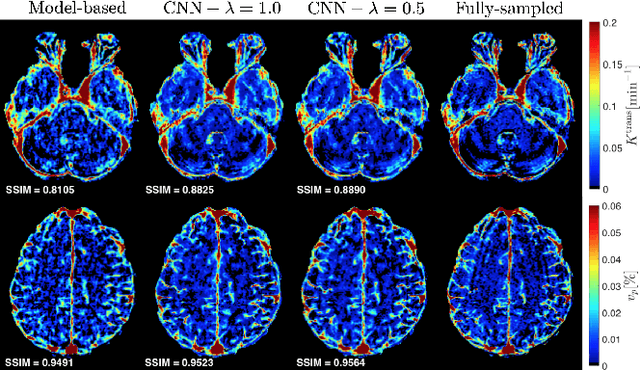
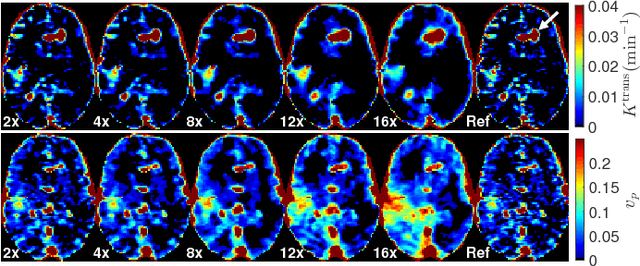
Abstract:Dynamic contrast-enhanced (DCE) MRI is an evolving imaging technique that provides a quantitative measure of pharmacokinetic (PK) parameters in body tissues, in which series of T1-weighted images are collected following the administration of a paramagnetic contrast agent. Unfortunately, in many applications, conventional clinical DCE-MRI suffers from low spatiotemporal resolution and insufficient volume coverage. In this paper, we propose a novel deep learning based approach to directly estimate the PK parameters from undersampled DCE-MRI data. Specifically, we design a custom loss function where we incorporate a forward physical model that relates the PK parameters to corrupted image-time series obtained due to subsampling in k-space. This allows the network to directly exploit the knowledge of true contrast agent kinetics in the training phase, and hence provide more accurate restoration of PK parameters. Experiments on clinical brain DCE datasets demonstrate the efficacy of our approach in terms of fidelity of PK parameter reconstruction and significantly faster parameter inference compared to a model-based iterative reconstruction method.
DeepASL: Kinetic Model Incorporated Loss for Denoising Arterial Spin Labeled MRI via Deep Residual Learning
Jun 12, 2018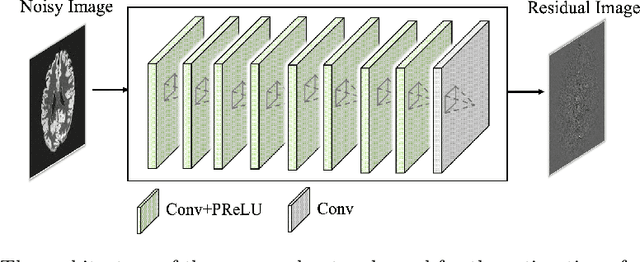
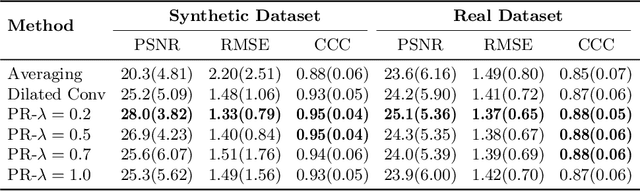
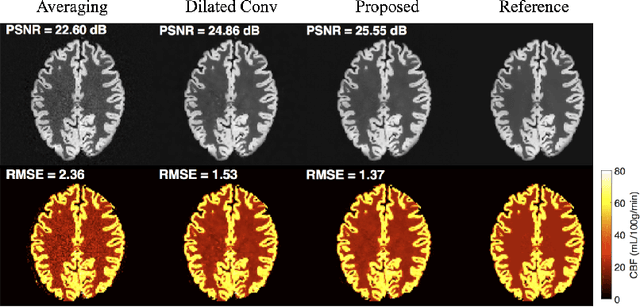
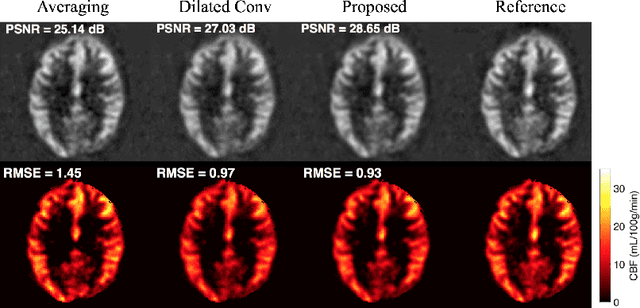
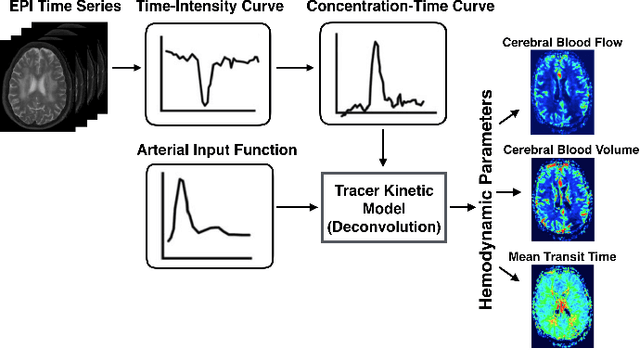
Abstract:Arterial spin labeling (ASL) allows to quantify the cerebral blood flow (CBF) by magnetic labeling of the arterial blood water. ASL is increasingly used in clinical studies due to its noninvasiveness, repeatability and benefits in quantification. However, ASL suffers from an inherently low-signal-to-noise ratio (SNR) requiring repeated measurements of control/spin-labeled (C/L) pairs to achieve a reasonable image quality, which in return increases motion sensitivity. This leads to clinically prolonged scanning times increasing the risk of motion artifacts. Thus, there is an immense need of advanced imaging and processing techniques in ASL. In this paper, we propose a novel deep learning based approach to improve the perfusion-weighted image quality obtained from a subset of all available pairwise C/L subtractions. Specifically, we train a deep fully convolutional network (FCN) to learn a mapping from noisy perfusion-weighted image and its subtraction (residual) from the clean image. Additionally, we incorporate the CBF estimation model in the loss function during training, which enables the network to produce high quality images while simultaneously enforcing the CBF estimates to be as close as reference CBF values. Extensive experiments on synthetic and clinical ASL datasets demonstrate the effectiveness of our method in terms of improved ASL image quality, accurate CBF parameter estimation and considerably small computation time during testing.
Accelerated Reconstruction of Perfusion-Weighted MRI Enforcing Jointly Local and Nonlocal Spatio-temporal Constraints
Aug 25, 2017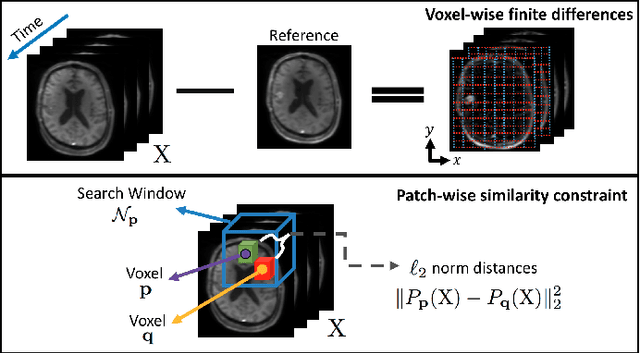
Abstract:Perfusion-weighted magnetic resonance imaging (MRI) is an imaging technique that allows one to measure tissue perfusion in an organ of interest through the injection of an intravascular paramagnetic contrast agent (CA). Due to a preference for high temporal and spatial resolution in many applications, this modality could significantly benefit from accelerated data acquisitions. In this paper, we specifically address the problem of reconstructing perfusion MR image series from a subset of k-space data. Our proposed approach is motivated by the observation that temporal variations (dynamics) in perfusion imaging often exhibit correlation across different spatial scales. Hence, we propose a model that jointly penalizes the voxel-wise deviations in temporal gradient images obtained based on a baseline, and the patch-wise dissimilarities between the spatio-temporal neighborhoods of entire image sequence. We validate our method on dynamic susceptibility contrast (DSC)-MRI and dynamic contrast-enhanced (DCE)-MRI brain perfusion datasets acquired from 10 tumor patients in total. We provide extensive analysis of reconstruction performance and perfusion parameter estimation in comparison to state-of-the-art reconstruction methods. Experimental results on clinical datasets demonstrate that our reconstruction model can potentially achieve up to 8-fold acceleration by enabling accurate estimation of perfusion parameters while preserving spatial image details and reconstructing the complete perfusion time-intensity curves (TICs).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge