Christine Preibisch
Motion-Robust T2* Quantification from Gradient Echo MRI with Physics-Informed Deep Learning
Feb 24, 2025Abstract:Purpose: T2* quantification from gradient echo magnetic resonance imaging is particularly affected by subject motion due to the high sensitivity to magnetic field inhomogeneities, which are influenced by motion and might cause signal loss. Thus, motion correction is crucial to obtain high-quality T2* maps. Methods: We extend our previously introduced learning-based physics-informed motion correction method, PHIMO, by utilizing acquisition knowledge to enhance the reconstruction performance for challenging motion patterns and increase PHIMO's robustness to varying strengths of magnetic field inhomogeneities across the brain. We perform comprehensive evaluations regarding motion detection accuracy and image quality for data with simulated and real motion. Results: Our extended version of PHIMO outperforms the learning-based baseline methods both qualitatively and quantitatively with respect to line detection and image quality. Moreover, PHIMO performs on-par with a conventional state-of-the-art motion correction method for T2* quantification from gradient echo MRI, which relies on redundant data acquisition. Conclusion: PHIMO's competitive motion correction performance, combined with a reduction in acquisition time by over 40% compared to the state-of-the-art method, make it a promising solution for motion-robust T2* quantification in research settings and clinical routine.
Physics-Informed Deep Learning for Motion-Corrected Reconstruction of Quantitative Brain MRI
Mar 13, 2024Abstract:We propose PHIMO, a physics-informed learning-based motion correction method tailored to quantitative MRI. PHIMO leverages information from the signal evolution to exclude motion-corrupted k-space lines from a data-consistent reconstruction. We demonstrate the potential of PHIMO for the application of T2* quantification from gradient echo MRI, which is particularly sensitive to motion due to its sensitivity to magnetic field inhomogeneities. A state-of-the-art technique for motion correction requires redundant acquisition of the k-space center, prolonging the acquisition. We show that PHIMO can detect and exclude intra-scan motion events and, thus, correct for severe motion artifacts. PHIMO approaches the performance of the state-of-the-art motion correction method, while substantially reducing the acquisition time by over 40%, facilitating clinical applicability. Our code is available at https://github.com/HannahEichhorn/PHIMO.
Deep Learning for Retrospective Motion Correction in MRI: A Comprehensive Review
May 11, 2023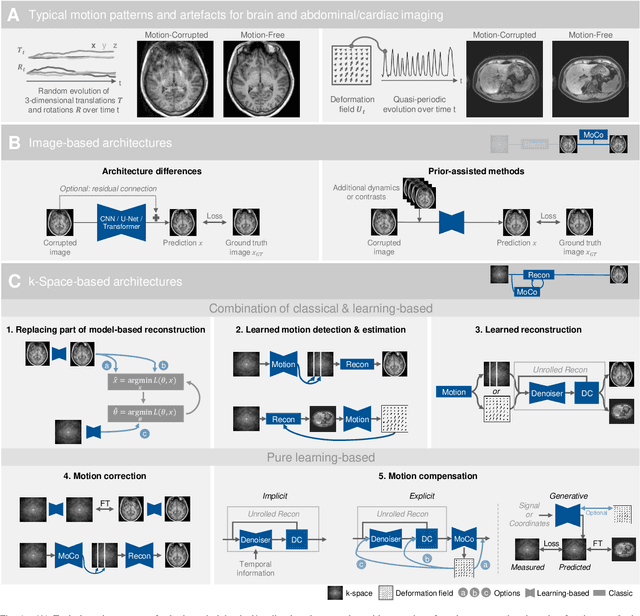
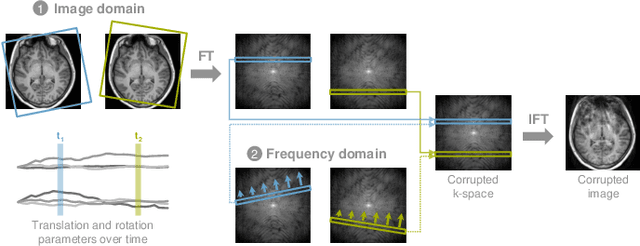
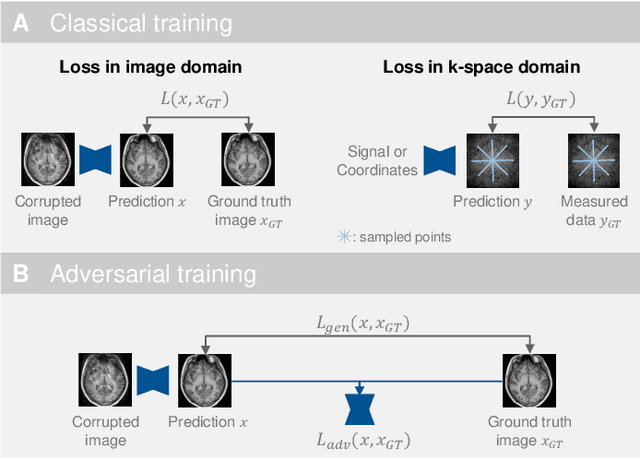
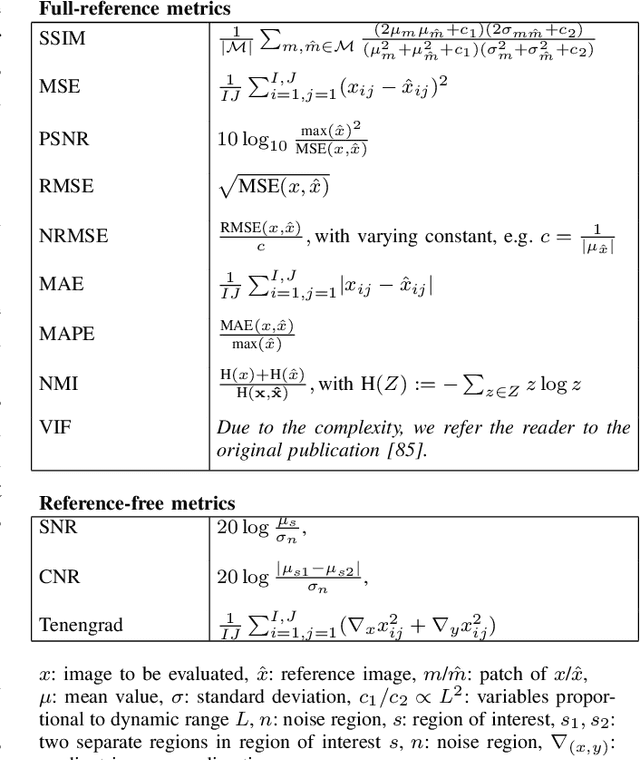
Abstract:Motion represents one of the major challenges in magnetic resonance imaging (MRI). Since the MR signal is acquired in frequency space, any motion of the imaged object leads to complex artefacts in the reconstructed image in addition to other MR imaging artefacts. Deep learning has been frequently proposed for motion correction at several stages of the reconstruction process. The wide range of MR acquisition sequences, anatomies and pathologies of interest, and motion patterns (rigid vs. deformable and random vs. regular) makes a comprehensive solution unlikely. To facilitate the transfer of ideas between different applications, this review provides a detailed overview of proposed methods for learning-based motion correction in MRI together with their common challenges and potentials. This review identifies differences and synergies in underlying data usage, architectures and evaluation strategies. We critically discuss general trends and outline future directions, with the aim to enhance interaction between different application areas and research fields.
Deep Learning-Based Detection of Motion-Affected k-Space Lines for T2*-Weighted MRI
Mar 20, 2023Abstract:T2*-weighted gradient echo MR imaging is strongly impacted by subject head motion due to motion-related changes in B0 inhomogeneities. Within the oxygenation-sensitive mqBOLD protocol, even mild motion during the acquisition of the T2*-weighted data propagates into errors in derived quantitative parameter maps. In order to correct these images without the need of repeated measurements, we propose to learn a classification of motion-affected k-space lines. To test this, we perform realistic motion simulations including motion-induced field inhomogeneity changes for supervised training. To detect the presence of motion in each phase encoding line, we train a convolutional neural network, leveraging the multi-echo information of the T2*-weighted images. The proposed network accurately detects motion-affected k-space lines for simulated displacements of $\geq$ 0.5mm (accuracy on test set: 92.5%). Finally, we show example reconstructions where we include these classification labels as weights in the data consistency term of an iterative reconstruction procedure, opening up exciting opportunities of k-space line detection in combination with more powerful reconstruction methods.
DeepASL: Kinetic Model Incorporated Loss for Denoising Arterial Spin Labeled MRI via Deep Residual Learning
Jun 12, 2018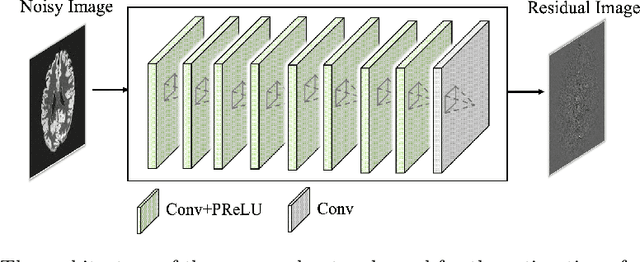
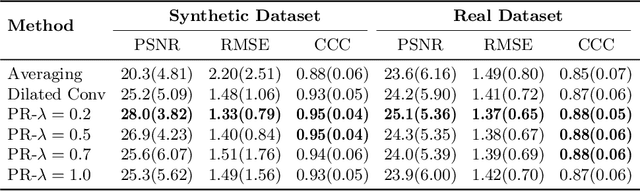
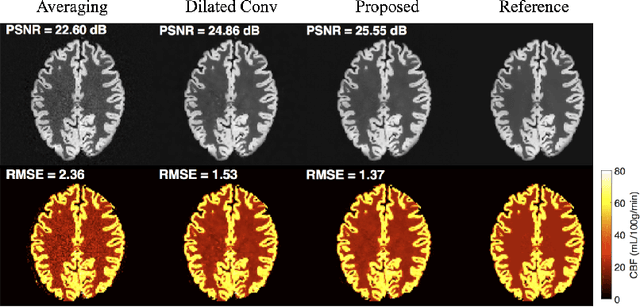
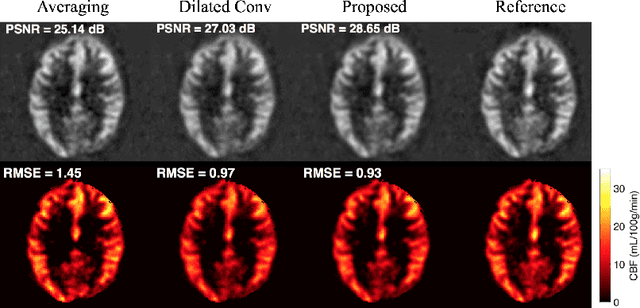
Abstract:Arterial spin labeling (ASL) allows to quantify the cerebral blood flow (CBF) by magnetic labeling of the arterial blood water. ASL is increasingly used in clinical studies due to its noninvasiveness, repeatability and benefits in quantification. However, ASL suffers from an inherently low-signal-to-noise ratio (SNR) requiring repeated measurements of control/spin-labeled (C/L) pairs to achieve a reasonable image quality, which in return increases motion sensitivity. This leads to clinically prolonged scanning times increasing the risk of motion artifacts. Thus, there is an immense need of advanced imaging and processing techniques in ASL. In this paper, we propose a novel deep learning based approach to improve the perfusion-weighted image quality obtained from a subset of all available pairwise C/L subtractions. Specifically, we train a deep fully convolutional network (FCN) to learn a mapping from noisy perfusion-weighted image and its subtraction (residual) from the clean image. Additionally, we incorporate the CBF estimation model in the loss function during training, which enables the network to produce high quality images while simultaneously enforcing the CBF estimates to be as close as reference CBF values. Extensive experiments on synthetic and clinical ASL datasets demonstrate the effectiveness of our method in terms of improved ASL image quality, accurate CBF parameter estimation and considerably small computation time during testing.
Accelerated Reconstruction of Perfusion-Weighted MRI Enforcing Jointly Local and Nonlocal Spatio-temporal Constraints
Aug 25, 2017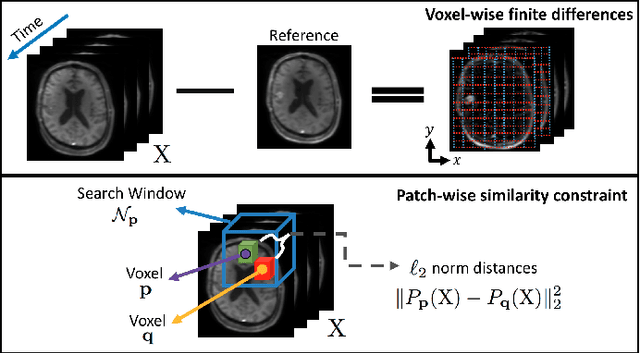
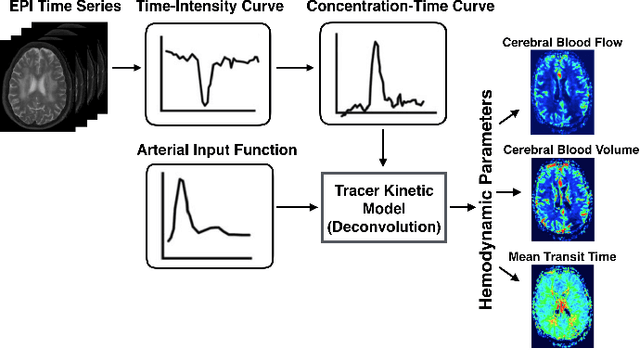
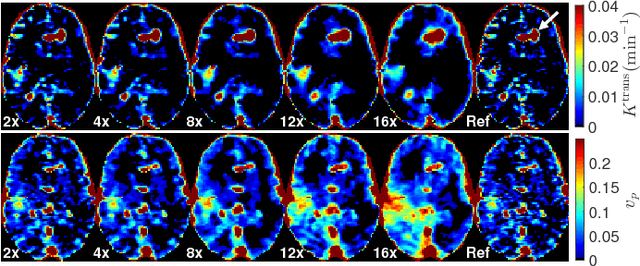
Abstract:Perfusion-weighted magnetic resonance imaging (MRI) is an imaging technique that allows one to measure tissue perfusion in an organ of interest through the injection of an intravascular paramagnetic contrast agent (CA). Due to a preference for high temporal and spatial resolution in many applications, this modality could significantly benefit from accelerated data acquisitions. In this paper, we specifically address the problem of reconstructing perfusion MR image series from a subset of k-space data. Our proposed approach is motivated by the observation that temporal variations (dynamics) in perfusion imaging often exhibit correlation across different spatial scales. Hence, we propose a model that jointly penalizes the voxel-wise deviations in temporal gradient images obtained based on a baseline, and the patch-wise dissimilarities between the spatio-temporal neighborhoods of entire image sequence. We validate our method on dynamic susceptibility contrast (DSC)-MRI and dynamic contrast-enhanced (DCE)-MRI brain perfusion datasets acquired from 10 tumor patients in total. We provide extensive analysis of reconstruction performance and perfusion parameter estimation in comparison to state-of-the-art reconstruction methods. Experimental results on clinical datasets demonstrate that our reconstruction model can potentially achieve up to 8-fold acceleration by enabling accurate estimation of perfusion parameters while preserving spatial image details and reconstructing the complete perfusion time-intensity curves (TICs).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge