Ponraj Chinnadurai
Automated Catheter Tip Repositioning for Intra-cardiac Echocardiography
Jan 21, 2022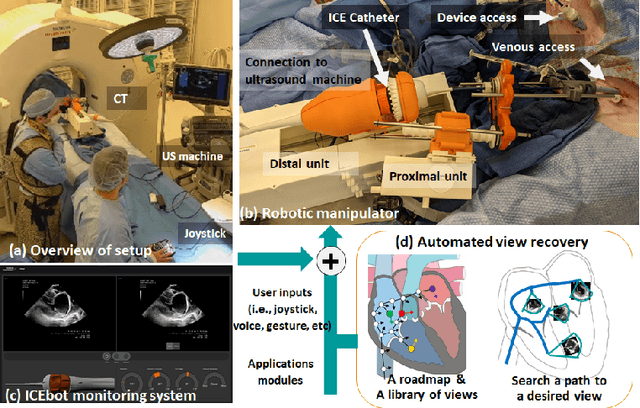

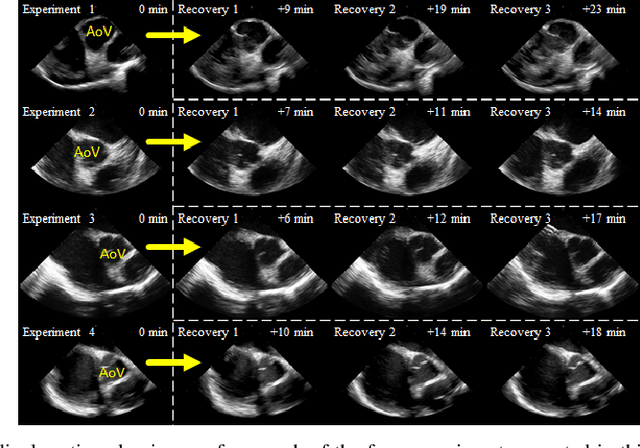
Abstract:Purpose: Intra-Cardiac Echocardiography (ICE) is a powerful imaging modality for guiding cardiac electrophysiology and structural heart interventions. ICE provides real-time observation of anatomy and devices, while enabling direct monitoring of potential complications. In single operator settings, the physician needs to switch back-and-forth between the ICE catheter and therapy device, making continuous ICE support impossible. Two operators setup are therefore sometimes implemented, with the challenge of increase room occupation and cost. Two operator setups are sometimes implemented, but increase procedural costs and room occupation. Methods: ICE catheter robotic control system is developed with automated catheter tip repositioning (i.e. view recovery) method, which can reproduce important views previously navigated to and saved by the user. The performance of the proposed method is demonstrated and evaluated in a combination of heart phantom and animal experiments. Results: Automated ICE view recovery achieved catheter tip position accuracy of 2.09 +/-0.90 mm and catheter image orientation accuracy of 3.93 +/- 2.07 degree in animal studies, and 0.67 +/- 0.79 mm and 0.37 +/- 0.19 degree in heart phantom studies, respectively. Our proposed method is also successfully used during transeptal puncture in animals without complications, showing the possibility for fluoro-less transeptal puncture with ICE catheter robot. Conclusion: Robotic ICE imaging has the potential to provide precise and reproducible anatomical views, which can reduce overall execution time, labor burden of procedures, and x-ray usage for a range of cardiac procedures. Keywords: Automated View Recovery, Path Planning, Intra-cardiac echocardiography (ICE), Catheter, Tendon-driven manipulator, Cardiac Imaging
Towards Automatic Manipulation of Intra-cardiac Echocardiography Catheter
Sep 12, 2020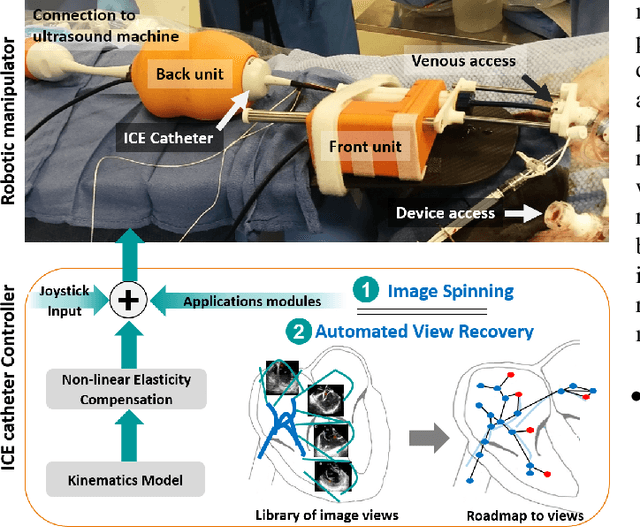
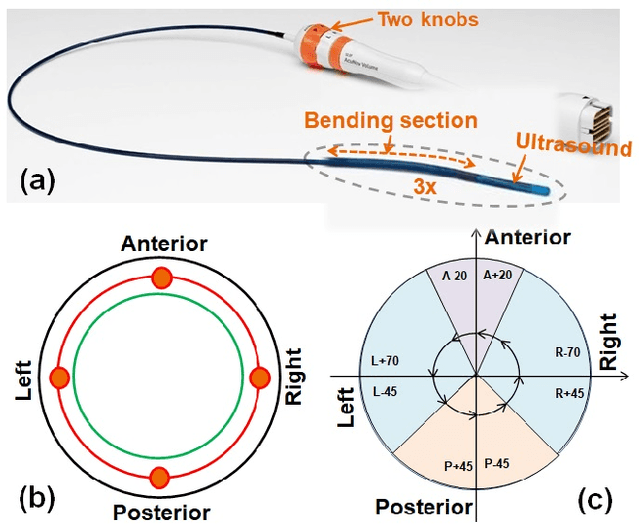
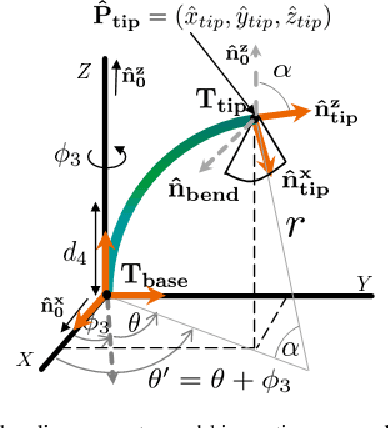
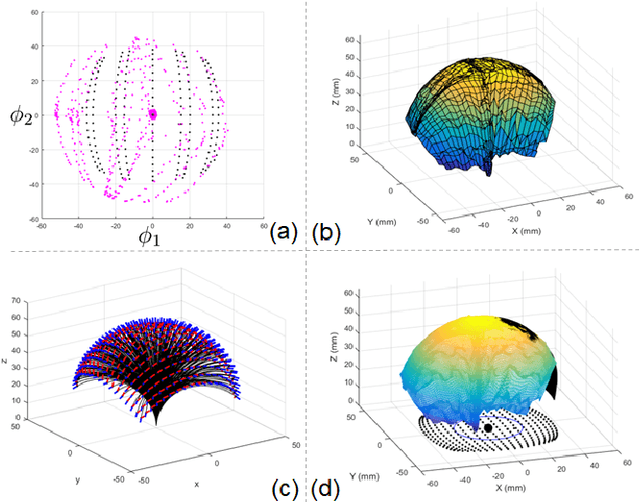
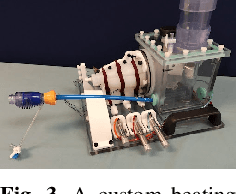
Abstract:Intra-cardiac Echocardiography (ICE) has been evolving as a real-time imaging modality of choice for guiding electrophiosology and structural heart interventions. ICE provides real-time imaging of anatomy, catheters, and complications such as pericardial effusion or thrombus formation. However, there now exists a high cognitive demand on physicians with the increased reliance on intraprocedural imaging. In response, we present a robotic manipulator for AcuNav ICE catheters to alleviate the physician's burden and support applied methods for more automated. Herein, we introduce two methods towards these goals: (1) a data-driven method to compensate kinematic model errors due to non-linear elasticity in catheter bending, providing more precise robotic control and (2) an automated image recovery process that allows physicians to bookmark images during intervention and automatically return with the push of a button. To validate our error compensation method, we demonstrate a complex rotation of the ultrasound imaging plane evaluated on benchtop. Automated view recovery is validated by repeated imaging of landmarks on benchtop and in vivo experiments with position- and image-based analysis. Results support that a robotic-assist system for more autonomous ICE can provide a safe and efficient tool, potentially reducing the execution time and allowing more complex procedures to become common place.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge