Angelos Karlas
Intelligent Virtual Sonographer (IVS): Enhancing Physician-Robot-Patient Communication
Jul 17, 2025Abstract:The advancement and maturity of large language models (LLMs) and robotics have unlocked vast potential for human-computer interaction, particularly in the field of robotic ultrasound. While existing research primarily focuses on either patient-robot or physician-robot interaction, the role of an intelligent virtual sonographer (IVS) bridging physician-robot-patient communication remains underexplored. This work introduces a conversational virtual agent in Extended Reality (XR) that facilitates real-time interaction between physicians, a robotic ultrasound system(RUS), and patients. The IVS agent communicates with physicians in a professional manner while offering empathetic explanations and reassurance to patients. Furthermore, it actively controls the RUS by executing physician commands and transparently relays these actions to the patient. By integrating LLM-powered dialogue with speech-to-text, text-to-speech, and robotic control, our system enhances the efficiency, clarity, and accessibility of robotic ultrasound acquisition. This work constitutes a first step toward understanding how IVS can bridge communication gaps in physician-robot-patient interaction, providing more control and therefore trust into physician-robot interaction while improving patient experience and acceptance of robotic ultrasound.
Synomaly Noise and Multi-Stage Diffusion: A Novel Approach for Unsupervised Anomaly Detection in Ultrasound Imaging
Nov 06, 2024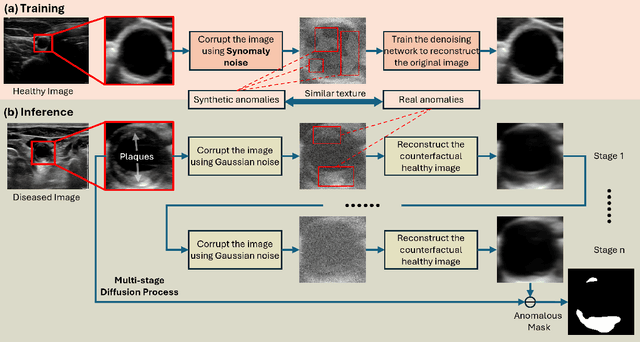
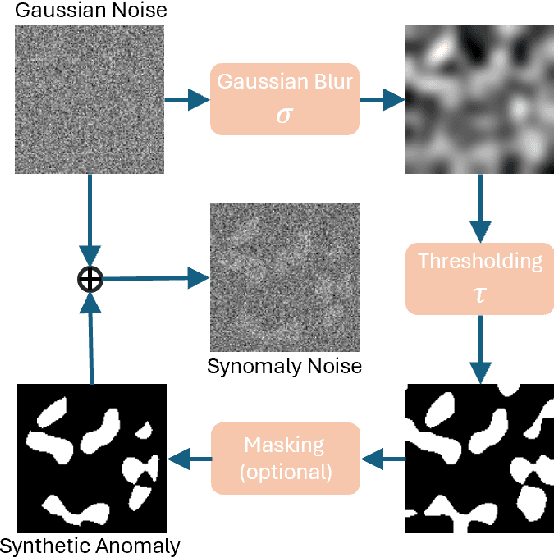
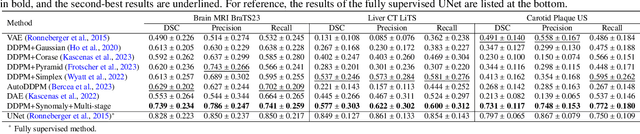
Abstract:Ultrasound (US) imaging is widely used in routine clinical practice due to its advantages of being radiation-free, cost-effective, and portable. However, the low reproducibility and quality of US images, combined with the scarcity of expert-level annotation, make the training of fully supervised segmentation models challenging. To address these issues, we propose a novel unsupervised anomaly detection framework based on a diffusion model that incorporates a synthetic anomaly (Synomaly) noise function and a multi-stage diffusion process. Synomaly noise introduces synthetic anomalies into healthy images during training, allowing the model to effectively learn anomaly removal. The multi-stage diffusion process is introduced to progressively denoise images, preserving fine details while improving the quality of anomaly-free reconstructions. The generated high-fidelity counterfactual healthy images can further enhance the interpretability of the segmentation models, as well as provide a reliable baseline for evaluating the extent of anomalies and supporting clinical decision-making. Notably, the unsupervised anomaly detection model is trained purely on healthy images, eliminating the need for anomalous training samples and pixel-level annotations. We validate the proposed approach on carotid US, brain MRI, and liver CT datasets. The experimental results demonstrate that the proposed framework outperforms existing state-of-the-art unsupervised anomaly detection methods, achieving performance comparable to fully supervised segmentation models in the US dataset. Additionally, ablation studies underline the importance of hyperparameter selection for Synomaly noise and the effectiveness of the multi-stage diffusion process in enhancing model performance.
A Method for Accurate Spatial Focusing Simulation via Numerical Integration and its Application in Optoacoustic Tomography
Sep 16, 2024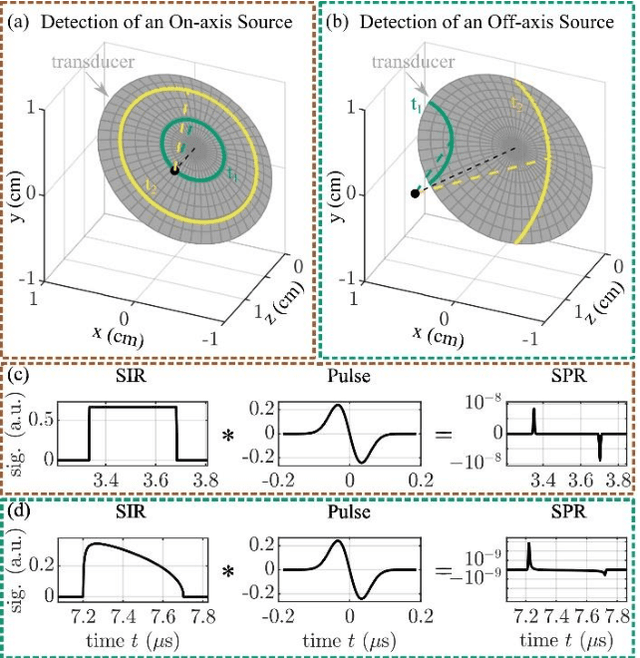
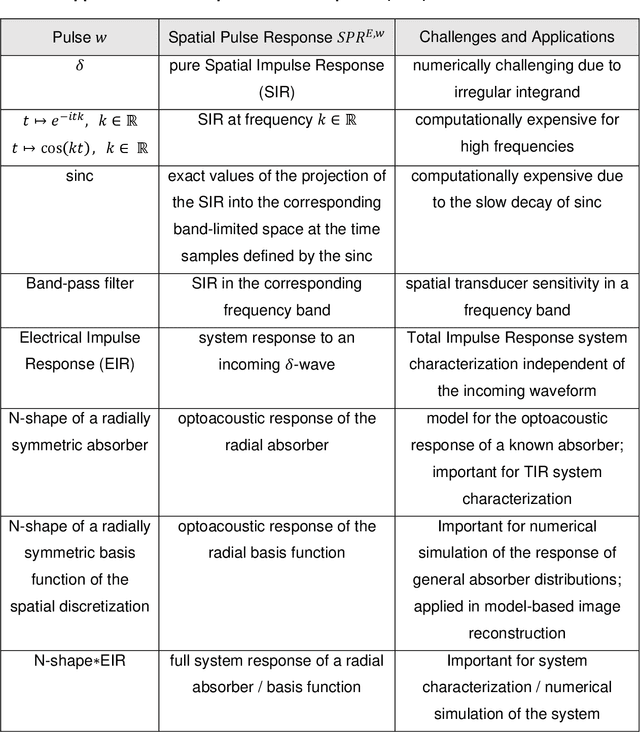
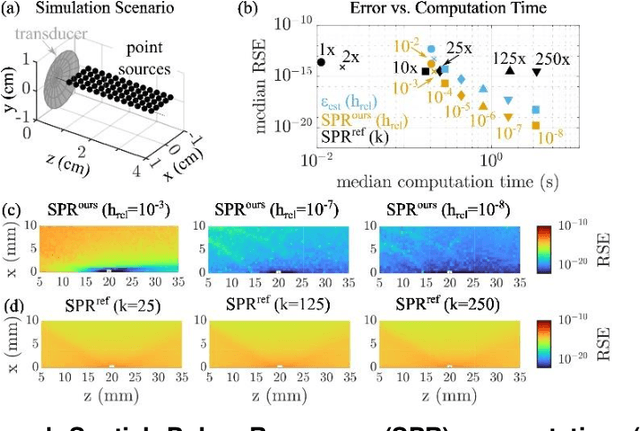
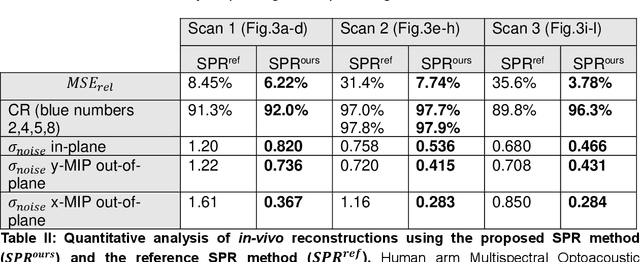
Abstract:The spatial sensitivity of an ultrasound transducer, which strongly influences its suitability for different applications, depends on the shape of the transducer surface. Accurate simulation of these spatial effects is important for transducer characterization and design, and for system response modelling in imaging applications. In optoacoustic imaging, broadband transducers are used to capitalize on the rich frequency content of the signals, but their usage makes highly accurate simulations with general wave equation solvers prohibitively memory- and time-intensive. Therefore, specialized tools for simulating the isolated spatial focusing properties described by the spatial impulse response (SIR) have been developed. However, the challenging numerics of the SIR and the necessity to convolve the SIR with the wave shape generated by the optoacoustic absorber to simulate the system response lead to numerical inaccuracies of SIR-based methods. In addition, the approximation error of these methods cannot be controlled a priori. To circumvent the problems associated with the explicit calculation of SIR, we propose directly computing the convolution of the required wave shape with the SIR, which we call the spatial pulse response (SPR). We demonstrate that by utilizing an h-adaptive cubature algorithm, SPR can be computed with significantly higher accuracy than an SIR-based reference method, and the approximation error can be controlled with a tolerance parameter. In addition, the integration of accurate SPR simulations into model-based optoacoustic image reconstruction is shown to improve image contrast and reduce noise artifacts. Precise system characterization and simulation leads to improved imaging performance, ultimately increasing the value of optoacoustic imaging systems for clinical applications.
PHOCUS: Physics-Based Deconvolution for Ultrasound Resolution Enhancement
Aug 07, 2024Abstract:Ultrasound is widely used in medical diagnostics allowing for accessible and powerful imaging but suffers from resolution limitations due to diffraction and the finite aperture of the imaging system, which restricts diagnostic use. The impulse function of an ultrasound imaging system is called the point spread function (PSF), which is convolved with the spatial distribution of reflectors in the image formation process. Recovering high-resolution reflector distributions by removing image distortions induced by the convolution process improves image clarity and detail. Conventionally, deconvolution techniques attempt to rectify the imaging system's dependent PSF, working directly on the radio-frequency (RF) data. However, RF data is often not readily accessible. Therefore, we introduce a physics-based deconvolution process using a modeled PSF, working directly on the more commonly available B-mode images. By leveraging Implicit Neural Representations (INRs), we learn a continuous mapping from spatial locations to their respective echogenicity values, effectively compensating for the discretized image space. Our contribution consists of a novel methodology for retrieving a continuous echogenicity map directly from a B-mode image through a differentiable physics-based rendering pipeline for ultrasound resolution enhancement. We qualitatively and quantitatively evaluate our approach on synthetic data, demonstrating improvements over traditional methods in metrics such as PSNR and SSIM. Furthermore, we show qualitative enhancements on an ultrasound phantom and an in-vivo acquisition of a carotid artery.
Needle Segmentation Using GAN: Restoring Thin Instrument Visibility in Robotic Ultrasound
Jul 25, 2024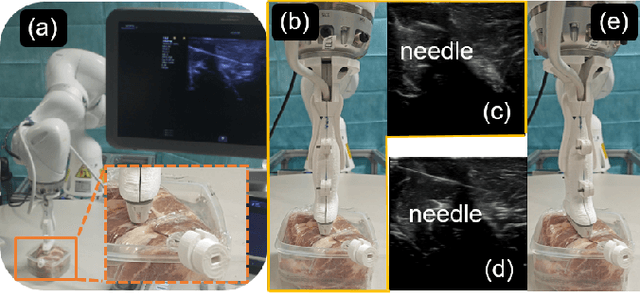
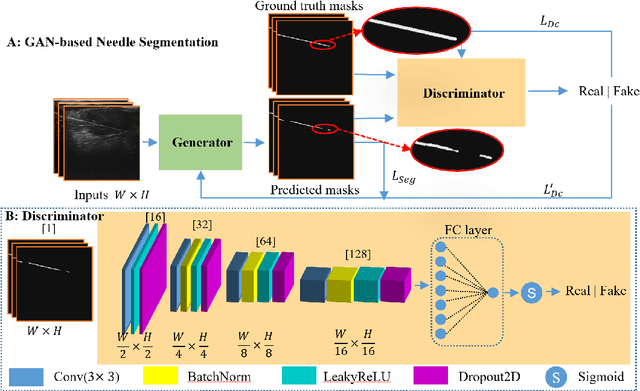
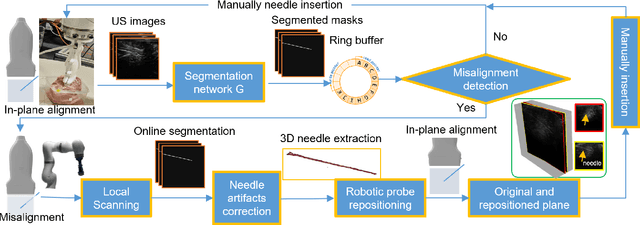
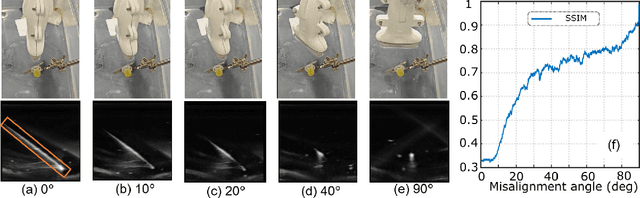
Abstract:Ultrasound-guided percutaneous needle insertion is a standard procedure employed in both biopsy and ablation in clinical practices. However, due to the complex interaction between tissue and instrument, the needle may deviate from the in-plane view, resulting in a lack of close monitoring of the percutaneous needle. To address this challenge, we introduce a robot-assisted ultrasound (US) imaging system designed to seamlessly monitor the insertion process and autonomously restore the visibility of the inserted instrument when misalignment happens. To this end, the adversarial structure is presented to encourage the generation of segmentation masks that align consistently with the ground truth in high-order space. This study also systematically investigates the effects on segmentation performance by exploring various training loss functions and their combinations. When misalignment between the probe and the percutaneous needle is detected, the robot is triggered to perform transverse searching to optimize the positional and rotational adjustment to restore needle visibility. The experimental results on ex-vivo porcine samples demonstrate that the proposed method can precisely segment the percutaneous needle (with a tip error of $0.37\pm0.29mm$ and an angle error of $1.19\pm 0.29^{\circ}$). Furthermore, the needle appearance can be successfully restored under the repositioned probe pose in all 45 trials, with repositioning errors of $1.51\pm0.95mm$ and $1.25\pm0.79^{\circ}$. from latex to text with math symbols
Invisible Needle Detection in Ultrasound: Leveraging Mechanism-Induced Vibration
Mar 21, 2024



Abstract:In clinical applications that involve ultrasound-guided intervention, the visibility of the needle can be severely impeded due to steep insertion and strong distractors such as speckle noise and anatomical occlusion. To address this challenge, we propose VibNet, a learning-based framework tailored to enhance the robustness and accuracy of needle detection in ultrasound images, even when the target becomes invisible to the naked eye. Inspired by Eulerian Video Magnification techniques, we utilize an external step motor to induce low-amplitude periodic motion on the needle. These subtle vibrations offer the potential to generate robust frequency features for detecting the motion patterns around the needle. To robustly and precisely detect the needle leveraging these vibrations, VibNet integrates learning-based Short-Time-Fourier-Transform and Hough-Transform modules to achieve successive sub-goals, including motion feature extraction in the spatiotemporal space, frequency feature aggregation, and needle detection in the Hough space. Based on the results obtained on distinct ex vivo porcine and bovine tissue samples, the proposed algorithm exhibits superior detection performance with efficient computation and generalization capability.
Robot-Assisted Deep Venous Thrombosis Ultrasound Examination using Virtual Fixture
Jan 04, 2024Abstract:Deep Venous Thrombosis (DVT) is a common vascular disease with blood clots inside deep veins, which may block blood flow or even cause a life-threatening pulmonary embolism. A typical exam for DVT using ultrasound (US) imaging is by pressing the target vein until its lumen is fully compressed. However, the compression exam is highly operator-dependent. To alleviate intra- and inter-variations, we present a robotic US system with a novel hybrid force motion control scheme ensuring position and force tracking accuracy, and soft landing of the probe onto the target surface. In addition, a path-based virtual fixture is proposed to realize easy human-robot interaction for repeat compression operation at the lesion location. To ensure the biometric measurements obtained in different examinations are comparable, the 6D scanning path is determined in a coarse-to-fine manner using both an external RGBD camera and US images. The RGBD camera is first used to extract a rough scanning path on the object. Then, the segmented vascular lumen from US images are used to optimize the scanning path to ensure the visibility of the target object. To generate a continuous scan path for developing virtual fixtures, an arc-length based path fitting model considering both position and orientation is proposed. Finally, the whole system is evaluated on a human-like arm phantom with an uneven surface.
MI-SegNet: Mutual Information-Based US Segmentation for Unseen Domain Generalization
Mar 22, 2023Abstract:Generalization capabilities of learning-based medical image segmentation across domains are currently limited by the performance degradation caused by the domain shift, particularly for ultrasound (US) imaging. The quality of US images heavily relies on carefully tuned acoustic parameters, which vary across sonographers, machines, and settings. To improve the generalizability on US images across domains, we propose MI-SegNet, a novel mutual information (MI) based framework to explicitly disentangle the anatomical and domain feature representations; therefore, robust domain-independent segmentation can be expected. Two encoders are employed to extract the relevant features for the disentanglement. The segmentation only uses the anatomical feature map for its prediction. In order to force the encoders to learn meaningful feature representations a cross-reconstruction method is used during training. Transformations, specific to either domain or anatomy are applied to guide the encoders in their respective feature extraction task. Additionally, any MI present in both feature maps is punished to further promote separate feature spaces. We validate the generalizability of the proposed domain-independent segmentation approach on several datasets with varying parameters and machines. Furthermore, we demonstrate the effectiveness of the proposed MI-SegNet serving as a pre-trained model by comparing it with state-of-the-art networks.
VesNet-RL: Simulation-based Reinforcement Learning for Real-World US Probe Navigation
May 10, 2022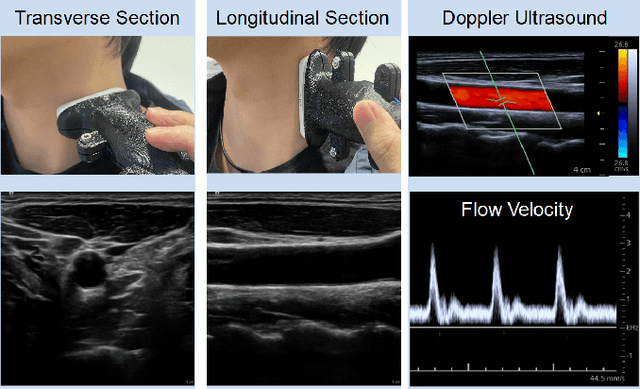
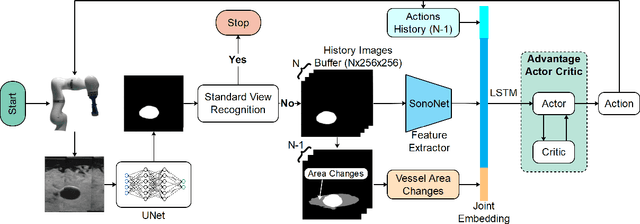
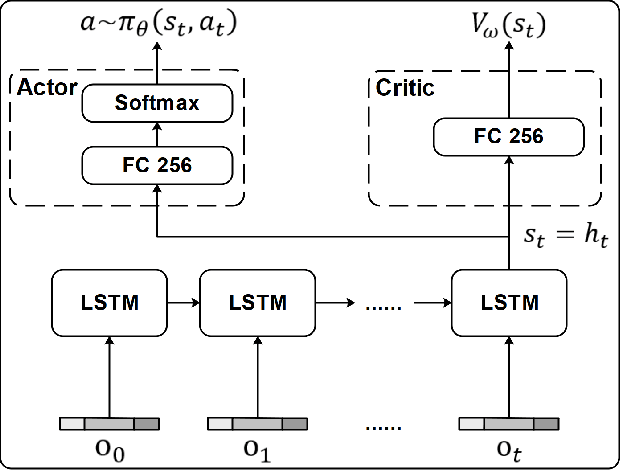

Abstract:Ultrasound (US) is one of the most common medical imaging modalities since it is radiation-free, low-cost, and real-time. In freehand US examinations, sonographers often navigate a US probe to visualize standard examination planes with rich diagnostic information. However, reproducibility and stability of the resulting images often suffer from intra- and inter-operator variation. Reinforcement learning (RL), as an interaction-based learning method, has demonstrated its effectiveness in visual navigating tasks; however, RL is limited in terms of generalization. To address this challenge, we propose a simulation-based RL framework for real-world navigation of US probes towards the standard longitudinal views of vessels. A UNet is used to provide binary masks from US images; thereby, the RL agent trained on simulated binary vessel images can be applied in real scenarios without further training. To accurately characterize actual states, a multi-modality state representation structure is introduced to facilitate the understanding of environments. Moreover, considering the characteristics of vessels, a novel standard view recognition approach based on the minimum bounding rectangle is proposed to terminate the searching process. To evaluate the effectiveness of the proposed method, the trained policy is validated virtually on 3D volumes of a volunteer's in-vivo carotid artery, and physically on custom-designed gel phantoms using robotic US. The results demonstrate that proposed approach can effectively and accurately navigate the probe towards the longitudinal view of vessels.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge