Felix Duelmer
UltrON: Ultrasound Occupancy Networks
Sep 10, 2025Abstract:In free-hand ultrasound imaging, sonographers rely on expertise to mentally integrate partial 2D views into 3D anatomical shapes. Shape reconstruction can assist clinicians in this process. Central to this task is the choice of shape representation, as it determines how accurately and efficiently the structure can be visualized, analyzed, and interpreted. Implicit representations, such as SDF and occupancy function, offer a powerful alternative to traditional voxel- or mesh-based methods by modeling continuous, smooth surfaces with compact storage, avoiding explicit discretization. Recent studies demonstrate that SDF can be effectively optimized using annotations derived from segmented B-mode ultrasound images. Yet, these approaches hinge on precise annotations, overlooking the rich acoustic information embedded in B-mode intensity. Moreover, implicit representation approaches struggle with the ultrasound's view-dependent nature and acoustic shadowing artifacts, which impair reconstruction. To address the problems resulting from occlusions and annotation dependency, we propose an occupancy-based representation and introduce \gls{UltrON} that leverages acoustic features to improve geometric consistency in weakly-supervised optimization regime. We show that these features can be obtained from B-mode images without additional annotation cost. Moreover, we propose a novel loss function that compensates for view-dependency in the B-mode images and facilitates occupancy optimization from multiview ultrasound. By incorporating acoustic properties, \gls{UltrON} generalizes to shapes of the same anatomy. We show that \gls{UltrON} mitigates the limitations of occlusions and sparse labeling and paves the way for more accurate 3D reconstruction. Code and dataset will be available at https://github.com/magdalena-wysocki/ultron.
UltraRay: Full-Path Ray Tracing for Enhancing Realism in Ultrasound Simulation
Jan 10, 2025



Abstract:Traditional ultrasound simulators solve the wave equation to model pressure distribution fields, achieving high accuracy but requiring significant computational time and resources. To address this, ray tracing approaches have been introduced, modeling wave propagation as rays interacting with boundaries and scatterers. However, existing models simplify ray propagation, generating echoes at interaction points without considering return paths to the sensor. This can result in unrealistic artifacts and necessitates careful scene tuning for plausible results. We propose a novel ultrasound simulation pipeline that utilizes a ray tracing algorithm to generate echo data, tracing each ray from the transducer through the scene and back to the sensor. To replicate advanced ultrasound imaging, we introduce a ray emission scheme optimized for plane wave imaging, incorporating delay and steering capabilities. Furthermore, we integrate a standard signal processing pipeline to simulate end-to-end ultrasound image formation. We showcase the efficacy of the proposed pipeline by modeling synthetic scenes featuring highly reflective objects, such as bones. In doing so, our proposed approach, UltraRay, not only enhances the overall visual quality but also improves the realism of the simulated images by accurately capturing secondary reflections and reducing unnatural artifacts. By building on top of a differentiable framework, the proposed pipeline lays the groundwork for a fast and differentiable ultrasound simulation tool necessary for gradient-based optimization, enabling advanced ultrasound beamforming strategies, neural network integration, and accurate inverse scene reconstruction.
PHOCUS: Physics-Based Deconvolution for Ultrasound Resolution Enhancement
Aug 07, 2024Abstract:Ultrasound is widely used in medical diagnostics allowing for accessible and powerful imaging but suffers from resolution limitations due to diffraction and the finite aperture of the imaging system, which restricts diagnostic use. The impulse function of an ultrasound imaging system is called the point spread function (PSF), which is convolved with the spatial distribution of reflectors in the image formation process. Recovering high-resolution reflector distributions by removing image distortions induced by the convolution process improves image clarity and detail. Conventionally, deconvolution techniques attempt to rectify the imaging system's dependent PSF, working directly on the radio-frequency (RF) data. However, RF data is often not readily accessible. Therefore, we introduce a physics-based deconvolution process using a modeled PSF, working directly on the more commonly available B-mode images. By leveraging Implicit Neural Representations (INRs), we learn a continuous mapping from spatial locations to their respective echogenicity values, effectively compensating for the discretized image space. Our contribution consists of a novel methodology for retrieving a continuous echogenicity map directly from a B-mode image through a differentiable physics-based rendering pipeline for ultrasound resolution enhancement. We qualitatively and quantitatively evaluate our approach on synthetic data, demonstrating improvements over traditional methods in metrics such as PSNR and SSIM. Furthermore, we show qualitative enhancements on an ultrasound phantom and an in-vivo acquisition of a carotid artery.
Machine Learning in Robotic Ultrasound Imaging: Challenges and Perspectives
Jan 04, 2024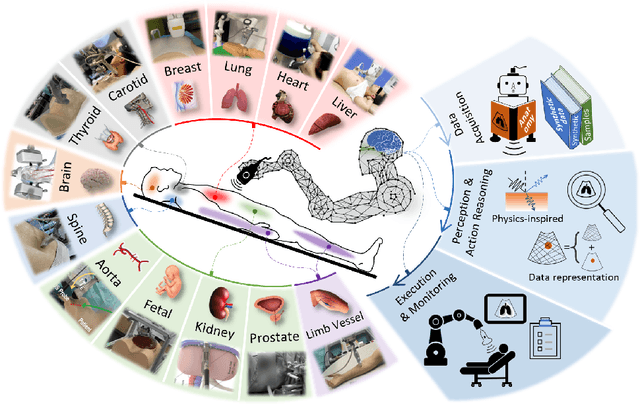
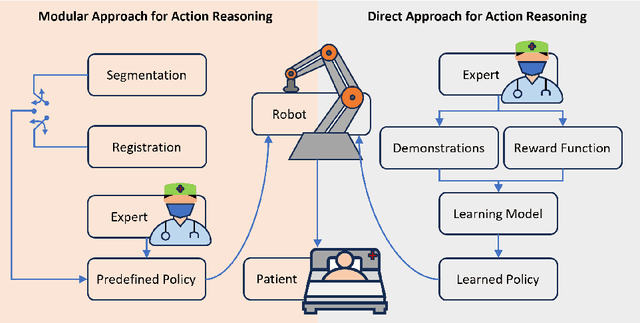
Abstract:This article reviews the recent advances in intelligent robotic ultrasound (US) imaging systems. We commence by presenting the commonly employed robotic mechanisms and control techniques in robotic US imaging, along with their clinical applications. Subsequently, we focus on the deployment of machine learning techniques in the development of robotic sonographers, emphasizing crucial developments aimed at enhancing the intelligence of these systems. The methods for achieving autonomous action reasoning are categorized into two sets of approaches: those relying on implicit environmental data interpretation and those using explicit interpretation. Throughout this exploration, we also discuss practical challenges, including those related to the scarcity of medical data, the need for a deeper understanding of the physical aspects involved, and effective data representation approaches. Moreover, we conclude by highlighting the open problems in the field and analyzing different possible perspectives on how the community could move forward in this research area.
DopUS-Net: Quality-Aware Robotic Ultrasound Imaging based on Doppler Signal
May 15, 2023Abstract:Medical ultrasound (US) is widely used to evaluate and stage vascular diseases, in particular for the preliminary screening program, due to the advantage of being radiation-free. However, automatic segmentation of small tubular structures (e.g., the ulnar artery) from cross-sectional US images is still challenging. To address this challenge, this paper proposes the DopUS-Net and a vessel re-identification module that leverage the Doppler effect to enhance the final segmentation result. Firstly, the DopUS-Net combines the Doppler images with B-mode images to increase the segmentation accuracy and robustness of small blood vessels. It incorporates two encoders to exploit the maximum potential of the Doppler signal and recurrent neural network modules to preserve sequential information. Input to the first encoder is a two-channel duplex image representing the combination of the grey-scale Doppler and B-mode images to ensure anatomical spatial correctness. The second encoder operates on the pure Doppler images to provide a region proposal. Secondly, benefiting from the Doppler signal, this work first introduces an online artery re-identification module to qualitatively evaluate the real-time segmentation results and automatically optimize the probe pose for enhanced Doppler images. This quality-aware module enables the closed-loop control of robotic screening to further improve the confidence and robustness of image segmentation. The experimental results demonstrate that the proposed approach with the re-identification process can significantly improve the accuracy and robustness of the segmentation results (dice score: from 0:54 to 0:86; intersection over union: from 0:47 to 0:78).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge