Zeli Chen
Anatomy-Aware Low-Dose CT Denoising via Pretrained Vision Models and Semantic-Guided Contrastive Learning
Aug 11, 2025Abstract:To reduce radiation exposure and improve the diagnostic efficacy of low-dose computed tomography (LDCT), numerous deep learning-based denoising methods have been developed to mitigate noise and artifacts. However, most of these approaches ignore the anatomical semantics of human tissues, which may potentially result in suboptimal denoising outcomes. To address this problem, we propose ALDEN, an anatomy-aware LDCT denoising method that integrates semantic features of pretrained vision models (PVMs) with adversarial and contrastive learning. Specifically, we introduce an anatomy-aware discriminator that dynamically fuses hierarchical semantic features from reference normal-dose CT (NDCT) via cross-attention mechanisms, enabling tissue-specific realism evaluation in the discriminator. In addition, we propose a semantic-guided contrastive learning module that enforces anatomical consistency by contrasting PVM-derived features from LDCT, denoised CT and NDCT, preserving tissue-specific patterns through positive pairs and suppressing artifacts via dual negative pairs. Extensive experiments conducted on two LDCT denoising datasets reveal that ALDEN achieves the state-of-the-art performance, offering superior anatomy preservation and substantially reducing over-smoothing issue of previous work. Further validation on a downstream multi-organ segmentation task (encompassing 117 anatomical structures) affirms the model's ability to maintain anatomical awareness.
UniReg: Foundation Model for Controllable Medical Image Registration
Mar 17, 2025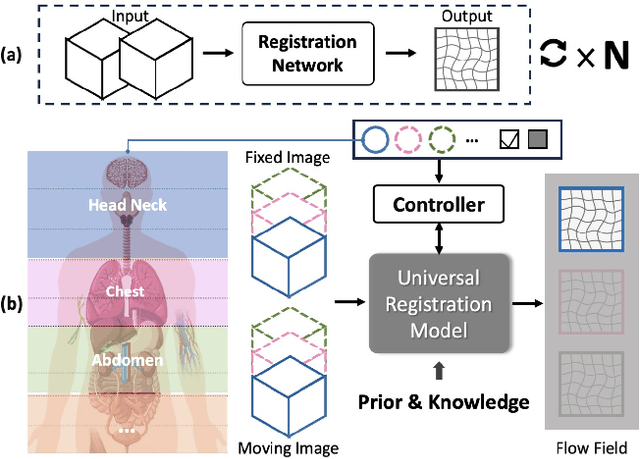
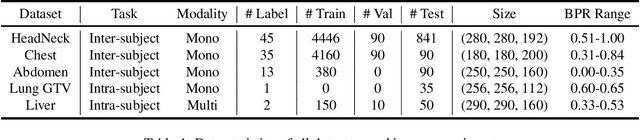
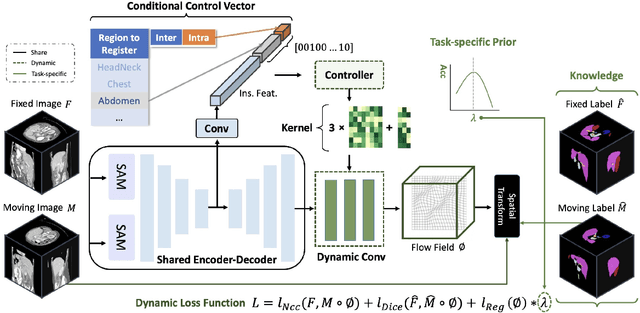
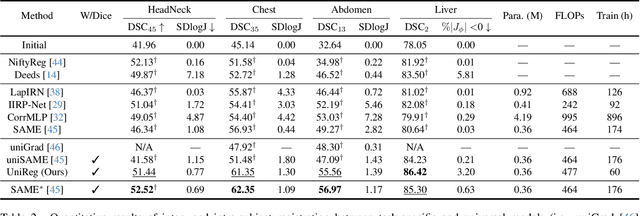
Abstract:Learning-based medical image registration has achieved performance parity with conventional methods while demonstrating a substantial advantage in computational efficiency. However, learning-based registration approaches lack generalizability across diverse clinical scenarios, requiring the laborious development of multiple isolated networks for specific registration tasks, e.g., inter-/intra-subject registration or organ-specific alignment. % To overcome this limitation, we propose \textbf{UniReg}, the first interactive foundation model for medical image registration, which combines the precision advantages of task-specific learning methods with the generalization of traditional optimization methods. Our key innovation is a unified framework for diverse registration scenarios, achieved through a conditional deformation field estimation within a unified registration model. This is realized through a dynamic learning paradigm that explicitly encodes: (1) anatomical structure priors, (2) registration type constraints (inter/intra-subject), and (3) instance-specific features, enabling the generation of scenario-optimal deformation fields. % Through comprehensive experiments encompassing $90$ anatomical structures at different body regions, our UniReg model demonstrates comparable performance with contemporary state-of-the-art methodologies while achieving ~50\% reduction in required training iterations relative to the conventional learning-based paradigm. This optimization contributes to a significant reduction in computational resources, such as training time. Code and model will be available.
Leveraging Semantic Asymmetry for Precise Gross Tumor Volume Segmentation of Nasopharyngeal Carcinoma in Planning CT
Nov 27, 2024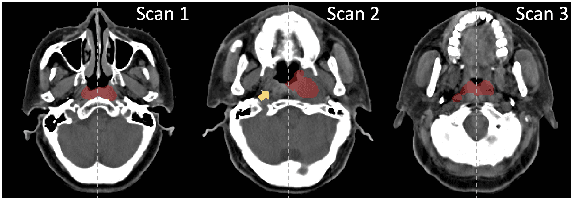
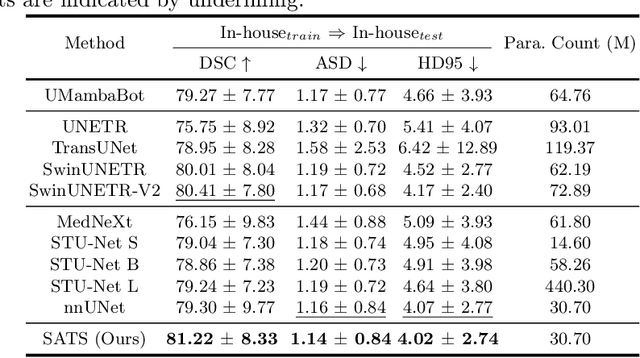
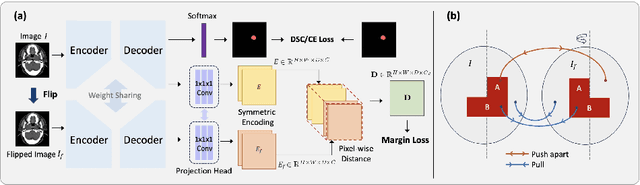
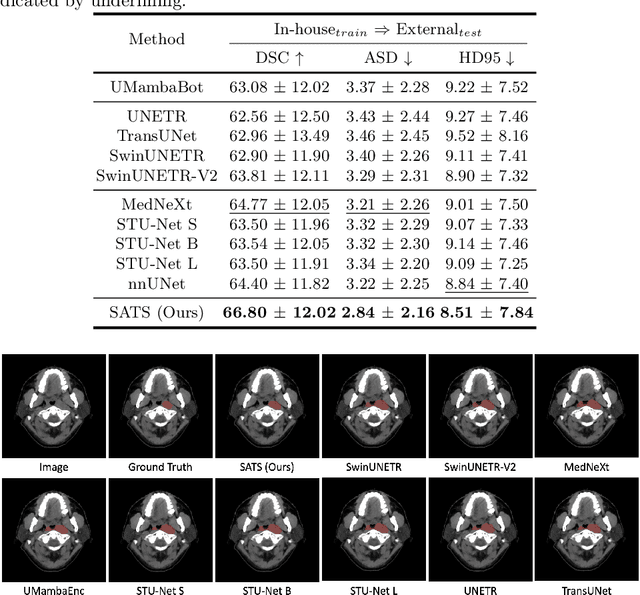
Abstract:In the radiation therapy of nasopharyngeal carcinoma (NPC), clinicians typically delineate the gross tumor volume (GTV) using non-contrast planning computed tomography to ensure accurate radiation dose delivery. However, the low contrast between tumors and adjacent normal tissues necessitates that radiation oncologists manually delineate the tumors, often relying on diagnostic MRI for guidance. % In this study, we propose a novel approach to directly segment NPC gross tumors on non-contrast planning CT images, circumventing potential registration errors when aligning MRI or MRI-derived tumor masks to planning CT. To address the low contrast issues between tumors and adjacent normal structures in planning CT, we introduce a 3D Semantic Asymmetry Tumor segmentation (SATs) method. Specifically, we posit that a healthy nasopharyngeal region is characteristically bilaterally symmetric, whereas the emergence of nasopharyngeal carcinoma disrupts this symmetry. Then, we propose a Siamese contrastive learning segmentation framework that minimizes the voxel-wise distance between original and flipped areas without tumor and encourages a larger distance between original and flipped areas with tumor. Thus, our approach enhances the sensitivity of features to semantic asymmetries. % Extensive experiments demonstrate that the proposed SATs achieves the leading NPC GTV segmentation performance in both internal and external testing, \emph{e.g.}, with at least 2\% absolute Dice score improvement and 12\% average distance error reduction when compared to other state-of-the-art methods in the external testing.
Boosting Medical Image Synthesis via Registration-guided Consistency and Disentanglement Learning
Jul 10, 2024



Abstract:Medical image synthesis remains challenging due to misalignment noise during training. Existing methods have attempted to address this challenge by incorporating a registration-guided module. However, these methods tend to overlook the task-specific constraints on the synthetic and registration modules, which may cause the synthetic module to still generate spatially aligned images with misaligned target images during training, regardless of the registration module's function. Therefore, this paper proposes registration-guided consistency and incorporates disentanglement learning for medical image synthesis. The proposed registration-guided consistency architecture fosters task-specificity within the synthetic and registration modules by applying identical deformation fields before and after synthesis, while enforcing output consistency through an alignment loss. Moreover, the synthetic module is designed to possess the capability of disentangling anatomical structures and specific styles across various modalities. An anatomy consistency loss is introduced to further compel the synthetic module to preserve geometrical integrity within latent spaces. Experiments conducted on both an in-house abdominal CECT-CT dataset and a publicly available pelvic MR-CT dataset have demonstrated the superiority of the proposed method.
DoseDiff: Distance-aware Diffusion Model for Dose Prediction in Radiotherapy
Jun 28, 2023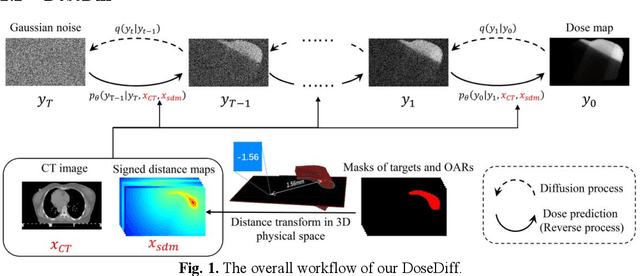
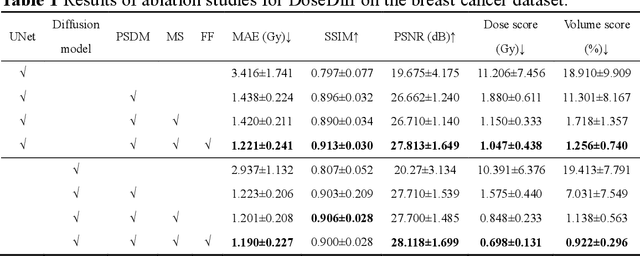
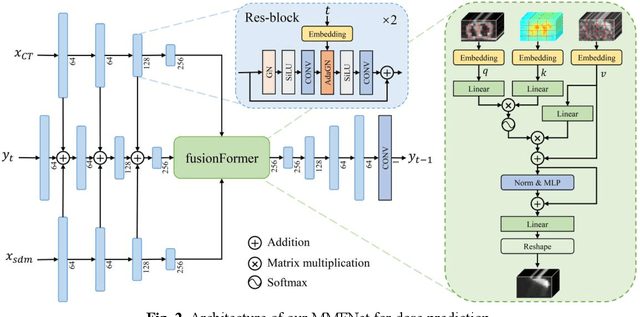
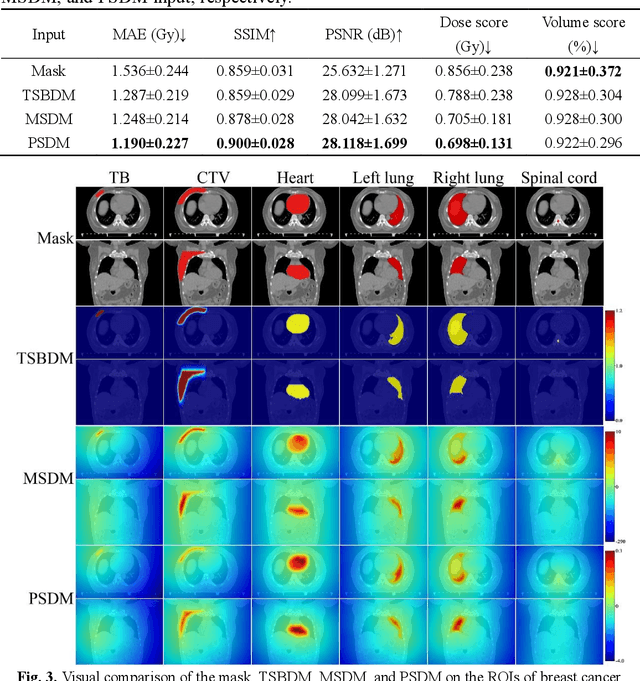
Abstract:Treatment planning is a critical component of the radiotherapy workflow, typically carried out by a medical physicist using a time-consuming trial-and-error manner. Previous studies have proposed knowledge-based or deep learning-based methods for predicting dose distribution maps to assist medical physicists in improving the efficiency of treatment planning. However, these dose prediction methods usuallylack the effective utilization of distance information between surrounding tissues andtargets or organs-at-risk (OARs). Moreover, they are poor in maintaining the distribution characteristics of ray paths in the predicted dose distribution maps, resulting in a loss of valuable information obtained by medical physicists. In this paper, we propose a distance-aware diffusion model (DoseDiff) for precise prediction of dose distribution. We define dose prediction as a sequence of denoising steps, wherein the predicted dose distribution map is generated with the conditions of the CT image and signed distance maps (SDMs). The SDMs are obtained by a distance transformation from the masks of targets or OARs, which provide the distance information from each pixel in the image to the outline of the targets or OARs. Besides, we propose a multiencoder and multi-scale fusion network (MMFNet) that incorporates a multi-scale fusion and a transformer-based fusion module to enhance information fusion between the CT image and SDMs at the feature level. Our model was evaluated on two datasets collected from patients with breast cancer and nasopharyngeal cancer, respectively. The results demonstrate that our DoseDiff outperforms the state-of-the-art dose prediction methods in terms of both quantitative and visual quality.
The state-of-the-art 3D anisotropic intracranial hemorrhage segmentation on non-contrast head CT: The INSTANCE challenge
Jan 12, 2023Abstract:Automatic intracranial hemorrhage segmentation in 3D non-contrast head CT (NCCT) scans is significant in clinical practice. Existing hemorrhage segmentation methods usually ignores the anisotropic nature of the NCCT, and are evaluated on different in-house datasets with distinct metrics, making it highly challenging to improve segmentation performance and perform objective comparisons among different methods. The INSTANCE 2022 was a grand challenge held in conjunction with the 2022 International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI). It is intended to resolve the above-mentioned problems and promote the development of both intracranial hemorrhage segmentation and anisotropic data processing. The INSTANCE released a training set of 100 cases with ground-truth and a validation set with 30 cases without ground-truth labels that were available to the participants. A held-out testing set with 70 cases is utilized for the final evaluation and ranking. The methods from different participants are ranked based on four metrics, including Dice Similarity Coefficient (DSC), Hausdorff Distance (HD), Relative Volume Difference (RVD) and Normalized Surface Dice (NSD). A total of 13 teams submitted distinct solutions to resolve the challenges, making several baseline models, pre-processing strategies and anisotropic data processing techniques available to future researchers. The winner method achieved an average DSC of 0.6925, demonstrating a significant growth over our proposed baseline method. To the best of our knowledge, the proposed INSTANCE challenge releases the first intracranial hemorrhage segmentation benchmark, and is also the first challenge that intended to resolve the anisotropic problem in 3D medical image segmentation, which provides new alternatives in these research fields.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge