Yinlong Xu
ProtFlow: Fast Protein Sequence Design via Flow Matching on Compressed Protein Language Model Embeddings
Apr 15, 2025Abstract:The design of protein sequences with desired functionalities is a fundamental task in protein engineering. Deep generative methods, such as autoregressive models and diffusion models, have greatly accelerated the discovery of novel protein sequences. However, these methods mainly focus on local or shallow residual semantics and suffer from low inference efficiency, large modeling space and high training cost. To address these challenges, we introduce ProtFlow, a fast flow matching-based protein sequence design framework that operates on embeddings derived from semantically meaningful latent space of protein language models. By compressing and smoothing the latent space, ProtFlow enhances performance while training on limited computational resources. Leveraging reflow techniques, ProtFlow enables high-quality single-step sequence generation. Additionally, we develop a joint design pipeline for the design scene of multichain proteins. We evaluate ProtFlow across diverse protein design tasks, including general peptides and long-chain proteins, antimicrobial peptides, and antibodies. Experimental results demonstrate that ProtFlow outperforms task-specific methods in these applications, underscoring its potential and broad applicability in computational protein sequence design and analysis.
MambaCapsule: Towards Transparent Cardiac Disease Diagnosis with Electrocardiography Using Mamba Capsule Network
Jul 30, 2024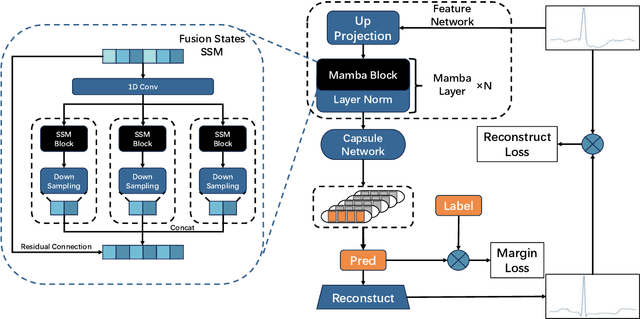

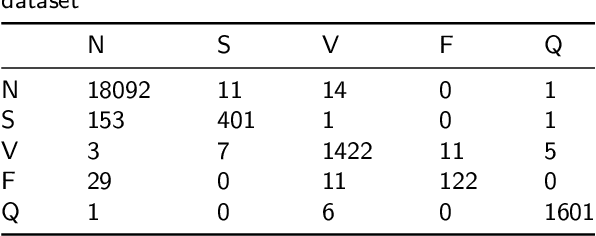
Abstract:Cardiac arrhythmia, a condition characterized by irregular heartbeats, often serves as an early indication of various heart ailments. With the advent of deep learning, numerous innovative models have been introduced for diagnosing arrhythmias using Electrocardiogram (ECG) signals. However, recent studies solely focus on the performance of models, neglecting the interpretation of their results. This leads to a considerable lack of transparency, posing a significant risk in the actual diagnostic process. To solve this problem, this paper introduces MambaCapsule, a deep neural networks for ECG arrhythmias classification, which increases the explainability of the model while enhancing the accuracy.Our model utilizes Mamba for feature extraction and Capsule networks for prediction, providing not only a confidence score but also signal features. Akin to the processing mechanism of human brain, the model learns signal features and their relationship between them by reconstructing ECG signals in the predicted selection. The model evaluation was conducted on MIT-BIH and PTB dataset, following the AAMI standard. MambaCapsule has achieved a total accuracy of 99.54% and 99.59% on the test sets respectively. These results demonstrate the promising performance of under the standard test protocol.
TWIN-GPT: Digital Twins for Clinical Trials via Large Language Model
Apr 01, 2024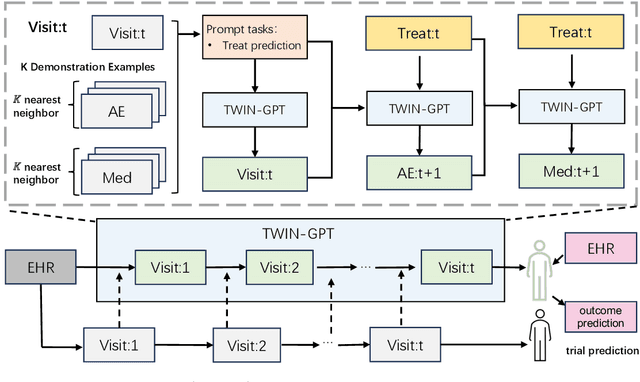


Abstract:Recently, there has been a burgeoning interest in virtual clinical trials, which simulate real-world scenarios and hold the potential to significantly enhance patient safety, expedite development, reduce costs, and contribute to the broader scientific knowledge in healthcare. Existing research often focuses on leveraging electronic health records (EHRs) to support clinical trial outcome prediction. Yet, trained with limited clinical trial outcome data, existing approaches frequently struggle to perform accurate predictions. Some research has attempted to generate EHRs to augment model development but has fallen short in personalizing the generation for individual patient profiles. Recently, the emergence of large language models has illuminated new possibilities, as their embedded comprehensive clinical knowledge has proven beneficial in addressing medical issues. In this paper, we propose a large language model-based digital twin creation approach, called TWIN-GPT. TWIN-GPT can establish cross-dataset associations of medical information given limited data, generating unique personalized digital twins for different patients, thereby preserving individual patient characteristics. Comprehensive experiments show that using digital twins created by TWIN-GPT can boost clinical trial outcome prediction, exceeding various previous prediction approaches. Besides, we also demonstrate that TWIN-GPT can generate high-fidelity trial data that closely approximate specific patients, aiding in more accurate result predictions in data-scarce situations. Moreover, our study provides practical evidence for the application of digital twins in healthcare, highlighting its potential significance.
Unraveling Babel: Exploring Multilingual Activation Patterns within Large Language Models
Feb 26, 2024Abstract:Recently, large language models (LLMs) have achieved tremendous breakthroughs in the field of language processing, yet their mechanisms in processing multiple languages remain agnostic. Therefore, in this work we study the multilingual activation patterns of LLMs. By transforming the original Large Language Models (LLMs) into a Mixture of Experts (MoE) architecture, we analyze the expert activation patterns when processing various languages and demonstrate the connections of these activation patterns at the level of language families. We discover the existence of non-language-specific neurons as well as language-specific activation neurons. Further exploration even showcases that merely leveraging high-frequency activation neurons can accelerate inference while maintaining comparable performance. These findings shed light on the LLMs' multilingual processing mechanism, and are of significant importance in guiding the multilingual training and model pruning of LLMs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge