Hanjing Zhou
ProtFlow: Fast Protein Sequence Design via Flow Matching on Compressed Protein Language Model Embeddings
Apr 15, 2025Abstract:The design of protein sequences with desired functionalities is a fundamental task in protein engineering. Deep generative methods, such as autoregressive models and diffusion models, have greatly accelerated the discovery of novel protein sequences. However, these methods mainly focus on local or shallow residual semantics and suffer from low inference efficiency, large modeling space and high training cost. To address these challenges, we introduce ProtFlow, a fast flow matching-based protein sequence design framework that operates on embeddings derived from semantically meaningful latent space of protein language models. By compressing and smoothing the latent space, ProtFlow enhances performance while training on limited computational resources. Leveraging reflow techniques, ProtFlow enables high-quality single-step sequence generation. Additionally, we develop a joint design pipeline for the design scene of multichain proteins. We evaluate ProtFlow across diverse protein design tasks, including general peptides and long-chain proteins, antimicrobial peptides, and antibodies. Experimental results demonstrate that ProtFlow outperforms task-specific methods in these applications, underscoring its potential and broad applicability in computational protein sequence design and analysis.
ProtCLIP: Function-Informed Protein Multi-Modal Learning
Dec 28, 2024Abstract:Multi-modality pre-training paradigm that aligns protein sequences and biological descriptions has learned general protein representations and achieved promising performance in various downstream applications. However, these works were still unable to replicate the extraordinary success of language-supervised visual foundation models due to the ineffective usage of aligned protein-text paired data and the lack of an effective function-informed pre-training paradigm. To address these issues, this paper curates a large-scale protein-text paired dataset called ProtAnno with a property-driven sampling strategy, and introduces a novel function-informed protein pre-training paradigm. Specifically, the sampling strategy determines selecting probability based on the sample confidence and property coverage, balancing the data quality and data quantity in face of large-scale noisy data. Furthermore, motivated by significance of the protein specific functional mechanism, the proposed paradigm explicitly model protein static and dynamic functional segments by two segment-wise pre-training objectives, injecting fine-grained information in a function-informed manner. Leveraging all these innovations, we develop ProtCLIP, a multi-modality foundation model that comprehensively represents function-aware protein embeddings. On 22 different protein benchmarks within 5 types, including protein functionality classification, mutation effect prediction, cross-modal transformation, semantic similarity inference and protein-protein interaction prediction, our ProtCLIP consistently achieves SOTA performance, with remarkable improvements of 75% on average in five cross-modal transformation benchmarks, 59.9% in GO-CC and 39.7% in GO-BP protein function prediction. The experimental results verify the extraordinary potential of ProtCLIP serving as the protein multi-modality foundation model.
S$^2$ALM: Sequence-Structure Pre-trained Large Language Model for Comprehensive Antibody Representation Learning
Nov 20, 2024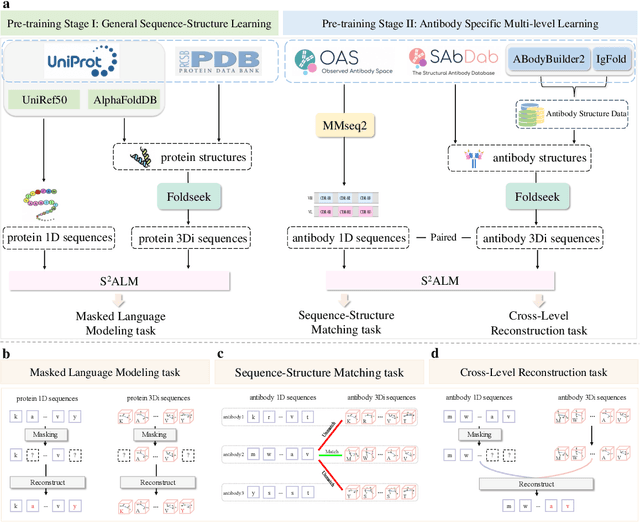
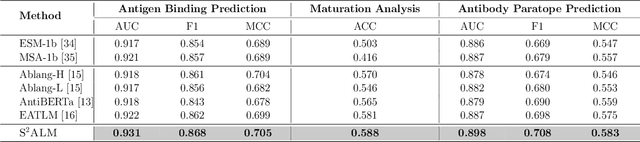
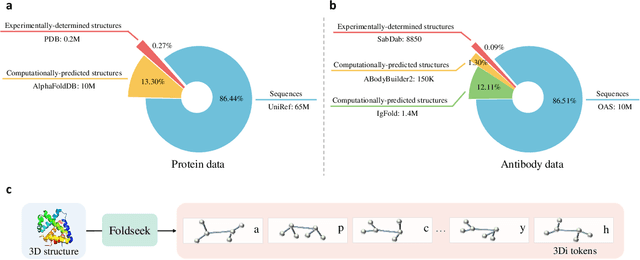
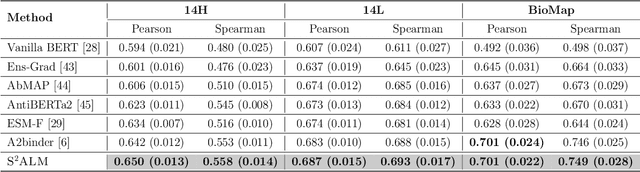
Abstract:Antibodies safeguard our health through their precise and potent binding to specific antigens, demonstrating promising therapeutic efficacy in the treatment of numerous diseases, including COVID-19. Recent advancements in biomedical language models have shown the great potential to interpret complex biological structures and functions. However, existing antibody specific models have a notable limitation that they lack explicit consideration for antibody structural information, despite the fact that both 1D sequence and 3D structure carry unique and complementary insights into antibody behavior and functionality. This paper proposes Sequence-Structure multi-level pre-trained Antibody Language Model (S$^2$ALM), combining holistic sequential and structural information in one unified, generic antibody foundation model. We construct a hierarchical pre-training paradigm incorporated with two customized multi-level training objectives to facilitate the modeling of comprehensive antibody representations. S$^2$ALM's representation space uncovers inherent functional binding mechanisms, biological evolution properties and structural interaction patterns. Pre-trained over 75 million sequences and 11.7 million structures, S$^2$ALM can be adopted for diverse downstream tasks: accurately predicting antigen-antibody binding affinities, precisely distinguishing B cell maturation stages, identifying antibody crucial binding positions, and specifically designing novel coronavirus-binding antibodies. Remarkably, S$^2$ALM outperforms well-established and renowned baselines and sets new state-of-the-art performance across extensive antibody specific understanding and generation tasks. S$^2$ALM's ability to model comprehensive and generalized representations further positions its potential to advance real-world therapeutic antibody development, potentially addressing unmet academic, industrial, and clinical needs.
Multi-Modal CLIP-Informed Protein Editing
Jul 27, 2024Abstract:Proteins govern most biological functions essential for life, but achieving controllable protein discovery and optimization remains challenging. Recently, machine learning-assisted protein editing (MLPE) has shown promise in accelerating optimization cycles and reducing experimental workloads. However, current methods struggle with the vast combinatorial space of potential protein edits and cannot explicitly conduct protein editing using biotext instructions, limiting their interactivity with human feedback. To fill these gaps, we propose a novel method called ProtET for efficient CLIP-informed protein editing through multi-modality learning. Our approach comprises two stages: in the pretraining stage, contrastive learning aligns protein-biotext representations encoded by two large language models (LLMs), respectively. Subsequently, during the protein editing stage, the fused features from editing instruction texts and original protein sequences serve as the final editing condition for generating target protein sequences. Comprehensive experiments demonstrated the superiority of ProtET in editing proteins to enhance human-expected functionality across multiple attribute domains, including enzyme catalytic activity, protein stability and antibody specific binding ability. And ProtET improves the state-of-the-art results by a large margin, leading to significant stability improvements of 16.67% and 16.90%. This capability positions ProtET to advance real-world artificial protein editing, potentially addressing unmet academic, industrial, and clinical needs.
Group-On: Boosting One-Shot Segmentation with Supportive Query
Apr 18, 2024Abstract:One-shot semantic segmentation aims to segment query images given only ONE annotated support image of the same class. This task is challenging because target objects in the support and query images can be largely different in appearance and pose (i.e., intra-class variation). Prior works suggested that incorporating more annotated support images in few-shot settings boosts performances but increases costs due to additional manual labeling. In this paper, we propose a novel approach for ONE-shot semantic segmentation, called Group-On, which packs multiple query images in batches for the benefit of mutual knowledge support within the same category. Specifically, after coarse segmentation masks of the batch of queries are predicted, query-mask pairs act as pseudo support data to enhance mask predictions mutually, under the guidance of a simple Group-On Voting module. Comprehensive experiments on three standard benchmarks show that, in the ONE-shot setting, our Group-On approach significantly outperforms previous works by considerable margins. For example, on the COCO-20i dataset, we increase mIoU scores by 8.21% and 7.46% on ASNet and HSNet baselines, respectively. With only one support image, Group-On can be even competitive with the counterparts using 5 annotated support images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge