Yaping Wu
Early and Prediagnostic Detection of Pancreatic Cancer from Computed Tomography
Jan 29, 2026Abstract:Pancreatic ductal adenocarcinoma (PDAC), one of the deadliest solid malignancies, is often detected at a late and inoperable stage. Retrospective reviews of prediagnostic CT scans, when conducted by expert radiologists aware that the patient later developed PDAC, frequently reveal lesions that were previously overlooked. To help detecting these lesions earlier, we developed an automated system named ePAI (early Pancreatic cancer detection with Artificial Intelligence). It was trained on data from 1,598 patients from a single medical center. In the internal test involving 1,009 patients, ePAI achieved an area under the receiver operating characteristic curve (AUC) of 0.939-0.999, a sensitivity of 95.3%, and a specificity of 98.7% for detecting small PDAC less than 2 cm in diameter, precisely localizing PDAC as small as 2 mm. In an external test involving 7,158 patients across 6 centers, ePAI achieved an AUC of 0.918-0.945, a sensitivity of 91.5%, and a specificity of 88.0%, precisely localizing PDAC as small as 5 mm. Importantly, ePAI detected PDACs on prediagnostic CT scans obtained 3 to 36 months before clinical diagnosis that had originally been overlooked by radiologists. It successfully detected and localized PDACs in 75 of 159 patients, with a median lead time of 347 days before clinical diagnosis. Our multi-reader study showed that ePAI significantly outperformed 30 board-certified radiologists by 50.3% (P < 0.05) in sensitivity while maintaining a comparable specificity of 95.4% in detecting PDACs early and prediagnostic. These findings suggest its potential of ePAI as an assistive tool to improve early detection of pancreatic cancer.
FD-MAR: Fourier Dual-domain Network for CT Metal Artifact Reduction
Jul 24, 2022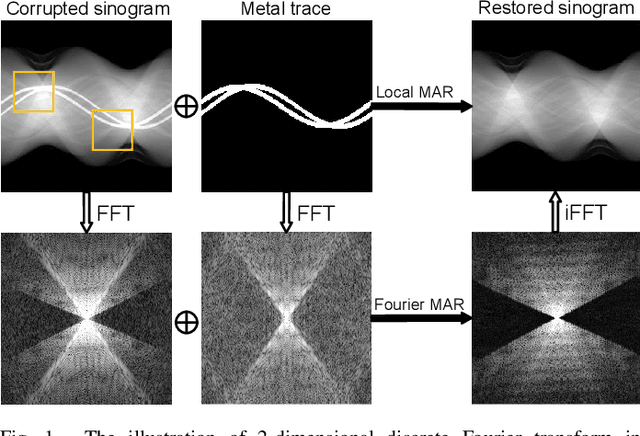
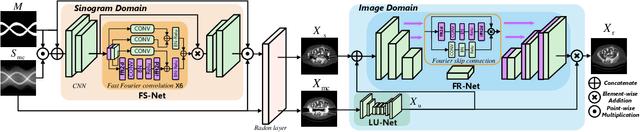
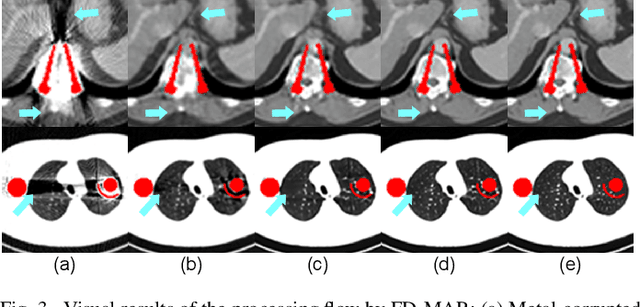
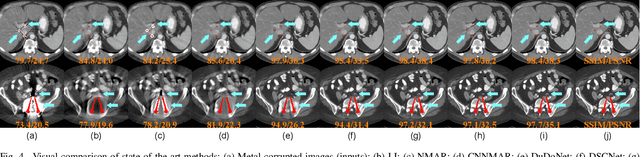
Abstract:The presence of high-density objects such as metal implants and dental fillings can introduce severely streak-like artifacts in computed tomography (CT) images, greatly limiting subsequent diagnosis. Although various deep neural networks-based methods have been proposed for metal artifact reduction (MAR), they usually suffer from poor performance due to limited exploitation of global context in the sinogram domain, secondary artifacts introduced in the image domain, and the requirement of precise metal masks. To address these issues, this paper explores fast Fourier convolution for MAR in both sinogram and image domains, and proposes a Fourier dual-domain network for MAR, termed FD-MAR. Specifically, we first propose a Fourier sinogram restoration network, which can leverage sinogram-wide receptive context to fill in the metal-corrupted region from uncorrupted region and, hence, is robust to the metal trace. Second, we propose a Fourier refinement network in the image domain, which can refine the reconstructed images in a local-to-global manner by exploring image-wide context information. As a result, the proposed FD-MAR can explore the sinogram- and image-wide receptive fields for MAR. By optimizing FD-MAR with a composite loss function, extensive experimental results demonstrate the superiority of the proposed FD-MAR over the state-of-the-art MAR methods in terms of quantitative metrics and visual comparison. Notably, FD-MAR does not require precise metal masks, which is of great importance in clinical routine.
AIDE: Annotation-efficient deep learning for automatic medical image segmentation
Dec 14, 2020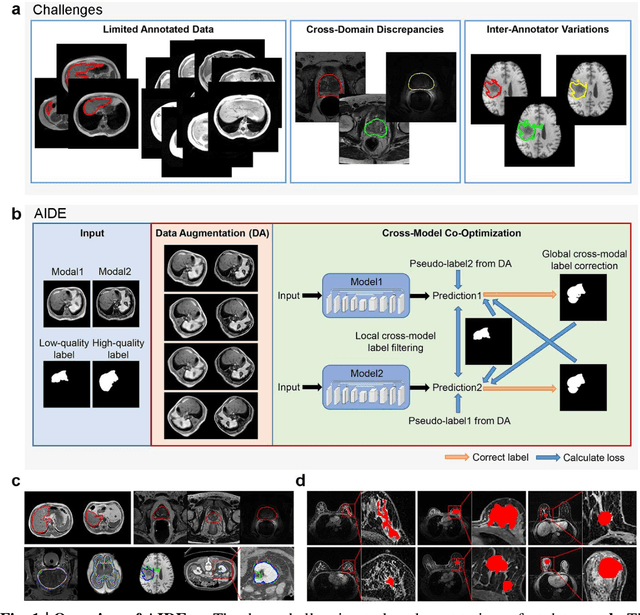
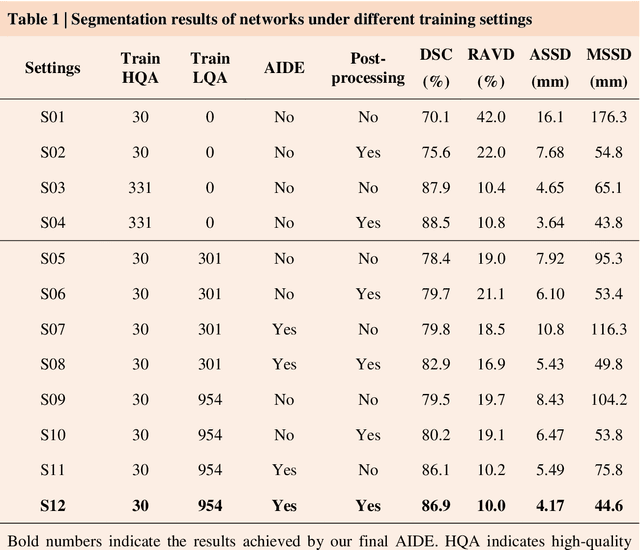
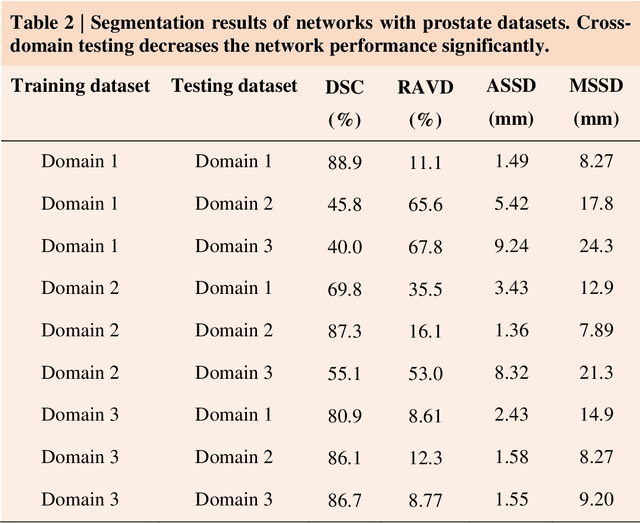
Abstract:Accurate image segmentation is crucial for medical imaging applications. The prevailing deep learning approaches typically rely on very large training datasets with high-quality manual annotations, which are often not available in medical imaging. We introduce Annotation-effIcient Deep lEarning (AIDE) to handle imperfect datasets with an elaborately designed cross-model self-correcting mechanism. AIDE improves the segmentation Dice scores of conventional deep learning models on open datasets possessing scarce or noisy annotations by up to 30%. For three clinical datasets containing 11,852 breast images of 872 patients from three medical centers, AIDE consistently produces segmentation maps comparable to those generated by the fully supervised counterparts as well as the manual annotations of independent radiologists by utilizing only 10% training annotations. Such a 10-fold improvement of efficiency in utilizing experts' labels has the potential to promote a wide range of biomedical applications.
Validation of a deep learning mammography model in a population with low screening rates
Nov 01, 2019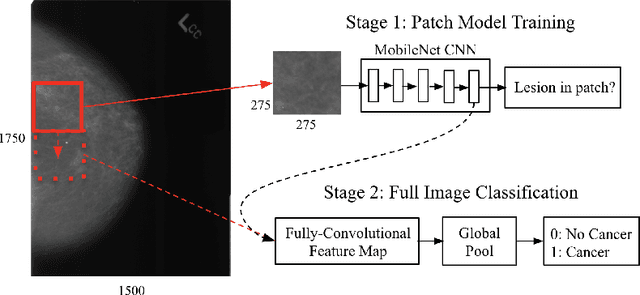
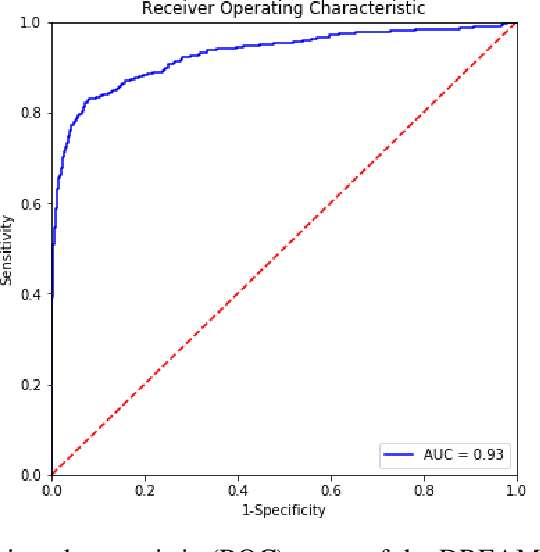
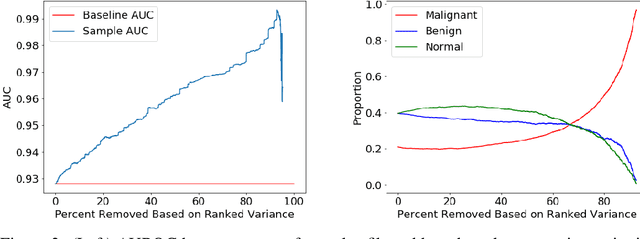
Abstract:A key promise of AI applications in healthcare is in increasing access to quality medical care in under-served populations and emerging markets. However, deep learning models are often only trained on data from advantaged populations that have the infrastructure and resources required for large-scale data collection. In this paper, we aim to empirically investigate the potential impact of such biases on breast cancer detection in mammograms. We specifically explore how a deep learning algorithm trained on screening mammograms from the US and UK generalizes to mammograms collected at a hospital in China, where screening is not widely implemented. For the evaluation, we use a top-scoring model developed for the Digital Mammography DREAM Challenge. Despite the change in institution and population composition, we find that the model generalizes well, exhibiting similar performance to that achieved in the DREAM Challenge, even when controlling for tumor size. We also illustrate a simple but effective method for filtering predictions based on model variance, which can be particularly useful for deployment in new settings. While there are many components in developing a clinically effective system, these results represent a promising step towards increasing access to life-saving screening mammography in populations where screening rates are currently low.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge