Xuedong Yuan
Confidence-aware multi-modality learning for eye disease screening
May 28, 2024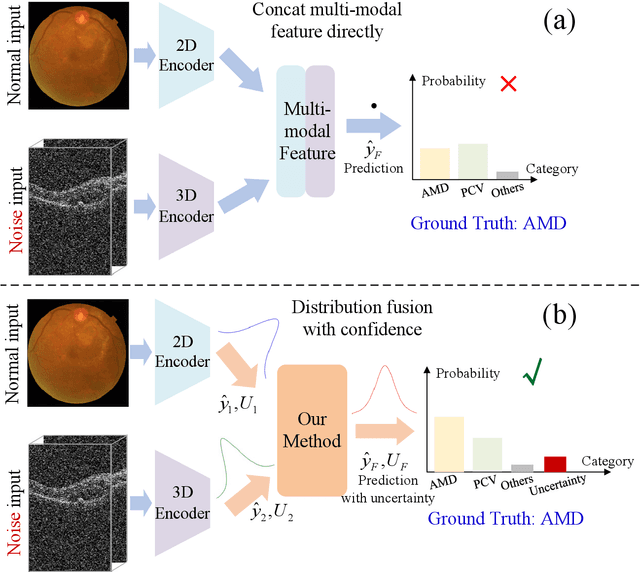
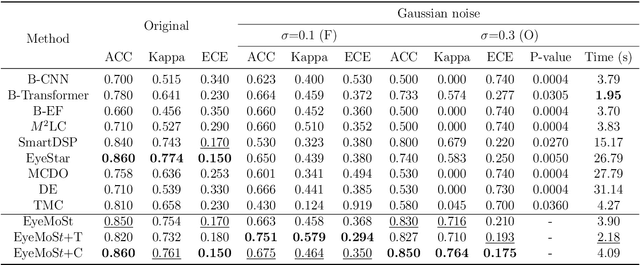
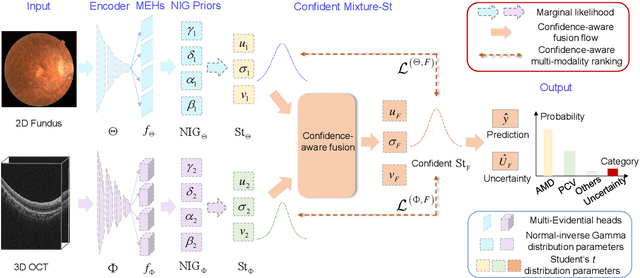
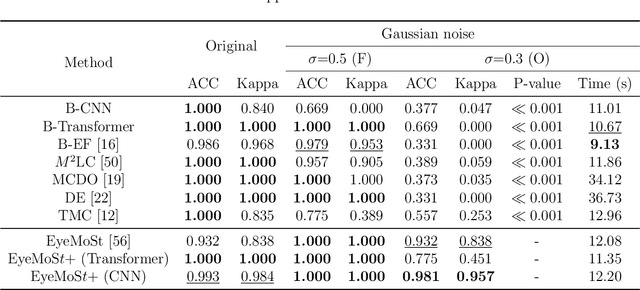
Abstract:Multi-modal ophthalmic image classification plays a key role in diagnosing eye diseases, as it integrates information from different sources to complement their respective performances. However, recent improvements have mainly focused on accuracy, often neglecting the importance of confidence and robustness in predictions for diverse modalities. In this study, we propose a novel multi-modality evidential fusion pipeline for eye disease screening. It provides a measure of confidence for each modality and elegantly integrates the multi-modality information using a multi-distribution fusion perspective. Specifically, our method first utilizes normal inverse gamma prior distributions over pre-trained models to learn both aleatoric and epistemic uncertainty for uni-modality. Then, the normal inverse gamma distribution is analyzed as the Student's t distribution. Furthermore, within a confidence-aware fusion framework, we propose a mixture of Student's t distributions to effectively integrate different modalities, imparting the model with heavy-tailed properties and enhancing its robustness and reliability. More importantly, the confidence-aware multi-modality ranking regularization term induces the model to more reasonably rank the noisy single-modal and fused-modal confidence, leading to improved reliability and accuracy. Experimental results on both public and internal datasets demonstrate that our model excels in robustness, particularly in challenging scenarios involving Gaussian noise and modality missing conditions. Moreover, our model exhibits strong generalization capabilities to out-of-distribution data, underscoring its potential as a promising solution for multimodal eye disease screening.
MedRG: Medical Report Grounding with Multi-modal Large Language Model
Apr 10, 2024



Abstract:Medical Report Grounding is pivotal in identifying the most relevant regions in medical images based on a given phrase query, a critical aspect in medical image analysis and radiological diagnosis. However, prevailing visual grounding approaches necessitate the manual extraction of key phrases from medical reports, imposing substantial burdens on both system efficiency and physicians. In this paper, we introduce a novel framework, Medical Report Grounding (MedRG), an end-to-end solution for utilizing a multi-modal Large Language Model to predict key phrase by incorporating a unique token, BOX, into the vocabulary to serve as an embedding for unlocking detection capabilities. Subsequently, the vision encoder-decoder jointly decodes the hidden embedding and the input medical image, generating the corresponding grounding box. The experimental results validate the effectiveness of MedRG, surpassing the performance of the existing state-of-the-art medical phrase grounding methods. This study represents a pioneering exploration of the medical report grounding task, marking the first-ever endeavor in this domain.
A Multi-view Impartial Decision Network for Frontotemporal Dementia Diagnosis
Jul 11, 2023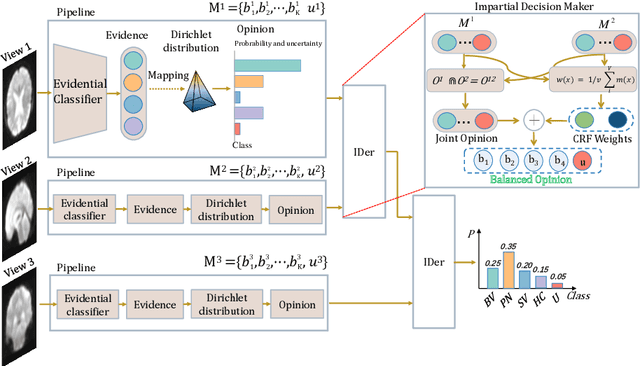
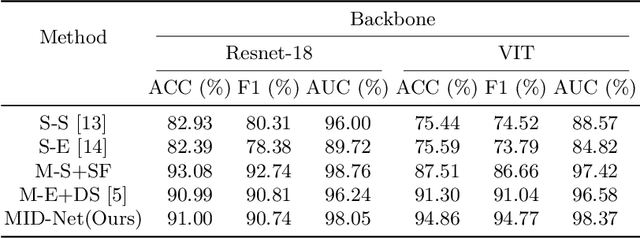
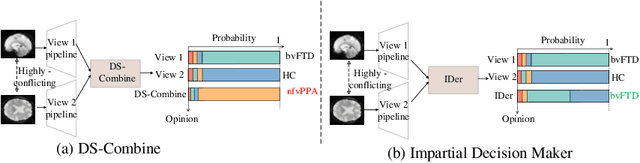
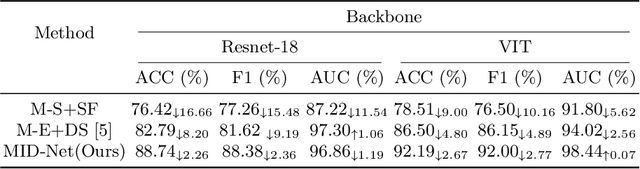
Abstract:Frontotemporal Dementia (FTD) diagnosis has been successfully progress using deep learning techniques. However, current FTD identification methods suffer from two limitations. Firstly, they do not exploit the potential of multi-view functional magnetic resonance imaging (fMRI) for classifying FTD. Secondly, they do not consider the reliability of the multi-view FTD diagnosis. To address these limitations, we propose a reliable multi-view impartial decision network (MID-Net) for FTD diagnosis in fMRI. Our MID-Net provides confidence for each view and generates a reliable prediction without any conflict. To achieve this, we employ multiple expert models to extract evidence from the abundant neural network information contained in fMRI images. We then introduce the Dirichlet Distribution to characterize the expert class probability distribution from an evidence level. Additionally, a novel Impartial Decision Maker (IDer) is proposed to combine the different opinions inductively to arrive at an unbiased prediction without additional computation cost. Overall, our MID-Net dynamically integrates the decisions of different experts on FTD disease, especially when dealing with multi-view high-conflict cases. Extensive experiments on a high-quality FTD fMRI dataset demonstrate that our model outperforms previous methods and provides high uncertainty for hard-to-classify examples. We believe that our approach represents a significant step toward the deployment of reliable FTD decision-making under multi-expert conditions. We will release the codes for reproduction after acceptance.
SAM-U: Multi-box prompts triggered uncertainty estimation for reliable SAM in medical image
Jul 11, 2023Abstract:Recently, Segmenting Anything has taken an important step towards general artificial intelligence. At the same time, its reliability and fairness have also attracted great attention, especially in the field of health care. In this study, we propose multi-box prompts triggered uncertainty estimation for SAM cues to demonstrate the reliability of segmented lesions or tissues. We estimate the distribution of SAM predictions via Monte Carlo with prior distribution parameters, which employs different prompts as formulation of test-time augmentation. Our experimental results found that multi-box prompts augmentation improve the SAM performance, and endowed each pixel with uncertainty. This provides the first paradigm for a reliable SAM.
Uncertainty-informed Mutual Learning for Joint Medical Image Classification and Segmentation
Mar 30, 2023



Abstract:Classification and segmentation are crucial in medical image analysis as they enable accurate diagnosis and disease monitoring. However, current methods often prioritize the mutual learning features and shared model parameters, while neglecting the reliability of features and performances. In this paper, we propose a novel Uncertainty-informed Mutual Learning (UML) framework for reliable and interpretable medical image analysis. Our UML introduces reliability to joint classification and segmentation tasks, leveraging mutual learning with uncertainty to improve performance. To achieve this, we first use evidential deep learning to provide image-level and pixel-wise confidences. Then, an Uncertainty Navigator Decoder is constructed for better using mutual features and generating segmentation results. Besides, an Uncertainty Instructor is proposed to screen reliable masks for classification. Overall, UML could produce confidence estimation in features and performance for each link (classification and segmentation). The experiments on the public datasets demonstrate that our UML outperforms existing methods in terms of both accuracy and robustness. Our UML has the potential to explore the development of more reliable and explainable medical image analysis models. We will release the codes for reproduction after acceptance.
Reliable Multimodality Eye Disease Screening via Mixture of Student's t Distributions
Mar 17, 2023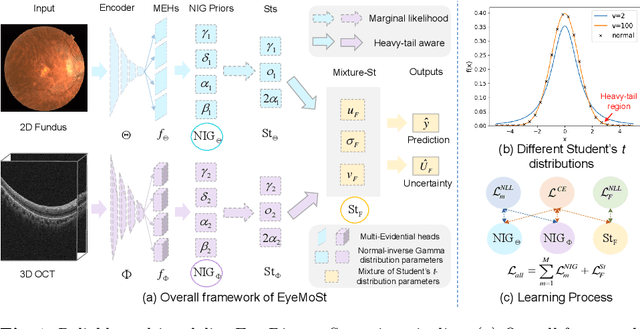
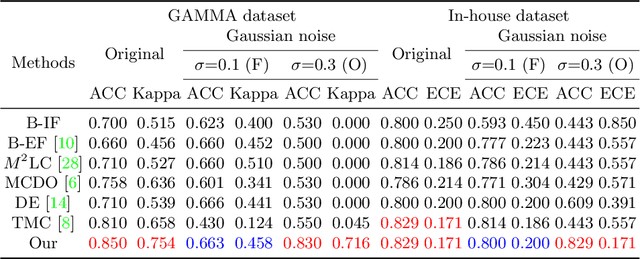
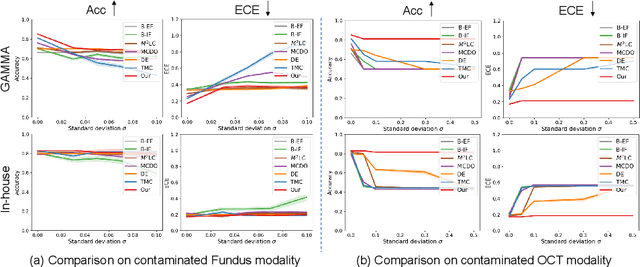

Abstract:Multimodality eye disease screening is crucial in ophthalmology as it integrates information from diverse sources to complement their respective performances. However, the existing methods are weak in assessing the reliability of each unimodality, and directly fusing an unreliable modality may cause screening errors. To address this issue, we introduce a novel multimodality evidential fusion pipeline for eye disease screening, EyeMoS$t$, which provides a measure of confidence for unimodality and elegantly integrates the multimodality information from a multi-distribution fusion perspective. Specifically, our model estimates both local uncertainty for unimodality and global uncertainty for the fusion modality to produce reliable classification results. More importantly, the proposed mixture of Student's $t$ distributions adaptively integrates different modalities to endow the model with heavy-tailed properties, increasing robustness and reliability. Our experimental findings on both public and in-house datasets show that our model is more reliable than current methods. Additionally, EyeMos$t$ has the potential ability to serve as a data quality discriminator, enabling reliable decision-making for multimodality eye disease screening.
A Review of Uncertainty Estimation and its Application in Medical Imaging
Feb 16, 2023



Abstract:The use of AI systems in healthcare for the early screening of diseases is of great clinical importance. Deep learning has shown great promise in medical imaging, but the reliability and trustworthiness of AI systems limit their deployment in real clinical scenes, where patient safety is at stake. Uncertainty estimation plays a pivotal role in producing a confidence evaluation along with the prediction of the deep model. This is particularly important in medical imaging, where the uncertainty in the model's predictions can be used to identify areas of concern or to provide additional information to the clinician. In this paper, we review the various types of uncertainty in deep learning, including aleatoric uncertainty, epistemic uncertainty, and out-of-distribution uncertainty, and we discuss how they can be estimated in medical imaging. We also review recent advances in deep learning models that incorporate uncertainty estimation in medical imaging. Finally, we discuss the challenges and future directions in uncertainty estimation in deep learning for medical imaging. We hope this review will ignite further interest in the community and provide researchers with an up-to-date reference regarding applications of uncertainty estimation models in medical imaging.
EvidenceCap: Towards trustworthy medical image segmentation via evidential identity cap
Jan 01, 2023



Abstract:Medical image segmentation (MIS) is essential for supporting disease diagnosis and treatment effect assessment. Despite considerable advances in artificial intelligence (AI) for MIS, clinicians remain skeptical of its utility, maintaining low confidence in such black box systems, with this problem being exacerbated by low generalization for out-of-distribution (OOD) data. To move towards effective clinical utilization, we propose a foundation model named EvidenceCap, which makes the box transparent in a quantifiable way by uncertainty estimation. EvidenceCap not only makes AI visible in regions of uncertainty and OOD data, but also enhances the reliability, robustness, and computational efficiency of MIS. Uncertainty is modeled explicitly through subjective logic theory to gather strong evidence from features. We show the effectiveness of EvidenceCap in three segmentation datasets and apply it to the clinic. Our work sheds light on clinical safe applications and explainable AI, and can contribute towards trustworthiness in the medical domain.
TBraTS: Trusted Brain Tumor Segmentation
Jun 30, 2022



Abstract:Despite recent improvements in the accuracy of brain tumor segmentation, the results still exhibit low levels of confidence and robustness. Uncertainty estimation is one effective way to change this situation, as it provides a measure of confidence in the segmentation results. In this paper, we propose a trusted brain tumor segmentation network which can generate robust segmentation results and reliable uncertainty estimations without excessive computational burden and modification of the backbone network. In our method, uncertainty is modeled explicitly using subjective logic theory, which treats the predictions of backbone neural network as subjective opinions by parameterizing the class probabilities of the segmentation as a Dirichlet distribution. Meanwhile, the trusted segmentation framework learns the function that gathers reliable evidence from the feature leading to the final segmentation results. Overall, our unified trusted segmentation framework endows the model with reliability and robustness to out-of-distribution samples. To evaluate the effectiveness of our model in robustness and reliability, qualitative and quantitative experiments are conducted on the BraTS 2019 dataset.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge