Walter Reade
TAU
Position: AI Competitions Provide the Gold Standard for Empirical Rigor in GenAI Evaluation
May 01, 2025Abstract:In this position paper, we observe that empirical evaluation in Generative AI is at a crisis point since traditional ML evaluation and benchmarking strategies are insufficient to meet the needs of evaluating modern GenAI models and systems. There are many reasons for this, including the fact that these models typically have nearly unbounded input and output spaces, typically do not have a well defined ground truth target, and typically exhibit strong feedback loops and prediction dependence based on context of previous model outputs. On top of these critical issues, we argue that the problems of {\em leakage} and {\em contamination} are in fact the most important and difficult issues to address for GenAI evaluations. Interestingly, the field of AI Competitions has developed effective measures and practices to combat leakage for the purpose of counteracting cheating by bad actors within a competition setting. This makes AI Competitions an especially valuable (but underutilized) resource. Now is time for the field to view AI Competitions as the gold standard for empirical rigor in GenAI evaluation, and to harness and harvest their results with according value.
Challenge design roadmap
Jan 15, 2024Abstract:Challenges can be seen as a type of game that motivates participants to solve serious tasks. As a result, competition organizers must develop effective game rules. However, these rules have multiple objectives beyond making the game enjoyable for participants. These objectives may include solving real-world problems, advancing scientific or technical areas, making scientific discoveries, and educating the public. In many ways, creating a challenge is similar to launching a product. It requires the same level of excitement and rigorous testing, and the goal is to attract ''customers'' in the form of participants. The process begins with a solid plan, such as a competition proposal that will eventually be submitted to an international conference and subjected to peer review. Although peer review does not guarantee quality, it does force organizers to consider the impact of their challenge, identify potential oversights, and generally improve its quality. This chapter provides guidelines for creating a strong plan for a challenge. The material draws on the preparation guidelines from organizations such as Kaggle 1 , ChaLearn 2 and Tailor 3 , as well as the NeurIPS proposal template, which some of the authors contributed to.
Predictive models of RNA degradation through dual crowdsourcing
Oct 14, 2021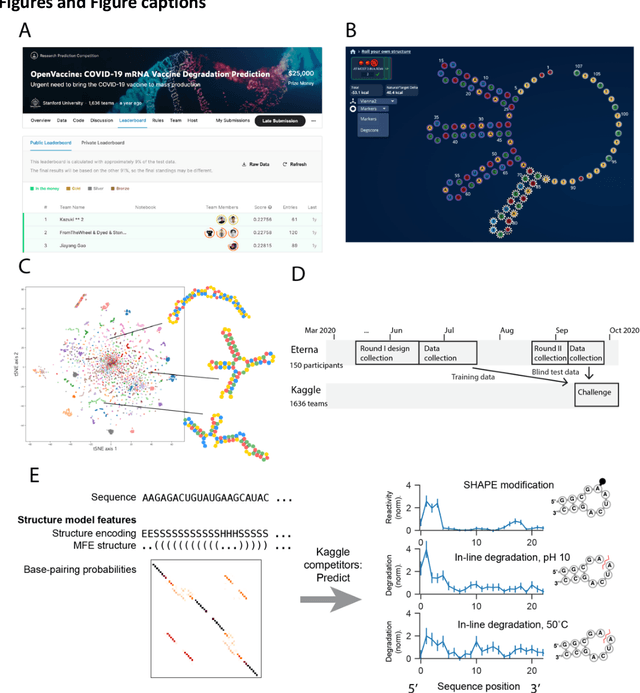
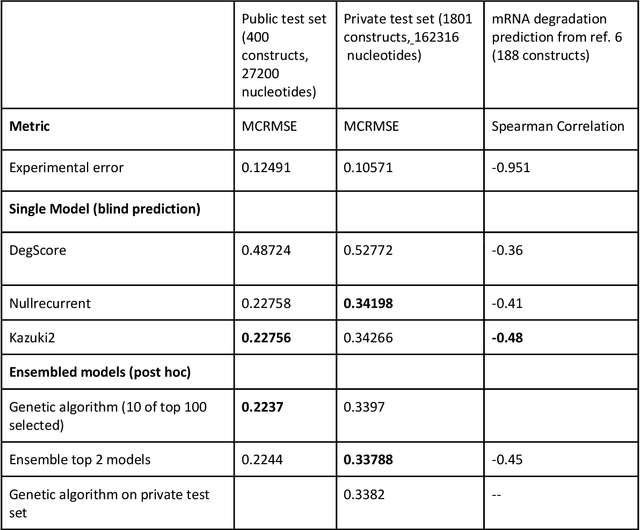
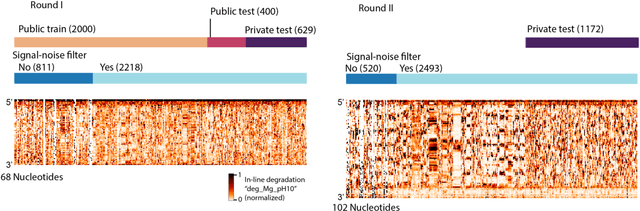
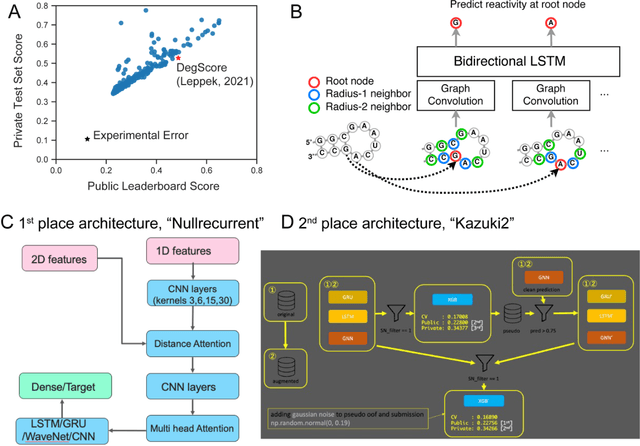
Abstract:Messenger RNA-based medicines hold immense potential, as evidenced by their rapid deployment as COVID-19 vaccines. However, worldwide distribution of mRNA molecules has been limited by their thermostability, which is fundamentally limited by the intrinsic instability of RNA molecules to a chemical degradation reaction called in-line hydrolysis. Predicting the degradation of an RNA molecule is a key task in designing more stable RNA-based therapeutics. Here, we describe a crowdsourced machine learning competition ("Stanford OpenVaccine") on Kaggle, involving single-nucleotide resolution measurements on 6043 102-130-nucleotide diverse RNA constructs that were themselves solicited through crowdsourcing on the RNA design platform Eterna. The entire experiment was completed in less than 6 months. Winning models demonstrated test set errors that were better by 50% than the previous state-of-the-art DegScore model. Furthermore, these models generalized to blindly predicting orthogonal degradation data on much longer mRNA molecules (504-1588 nucleotides) with improved accuracy over DegScore and other models. Top teams integrated natural language processing architectures and data augmentation techniques with predictions from previous dynamic programming models for RNA secondary structure. These results indicate that such models are capable of representing in-line hydrolysis with excellent accuracy, supporting their use for designing stabilized messenger RNAs. The integration of two crowdsourcing platforms, one for data set creation and another for machine learning, may be fruitful for other urgent problems that demand scientific discovery on rapid timescales.
The RSNA-ASNR-MICCAI BraTS 2021 Benchmark on Brain Tumor Segmentation and Radiogenomic Classification
Jul 05, 2021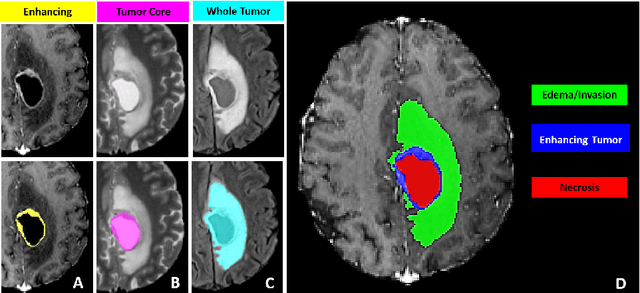
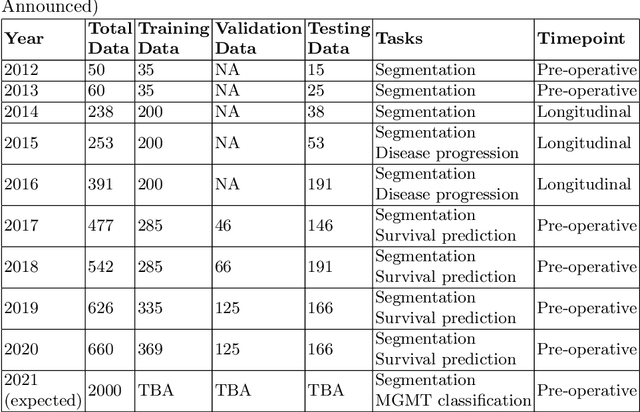
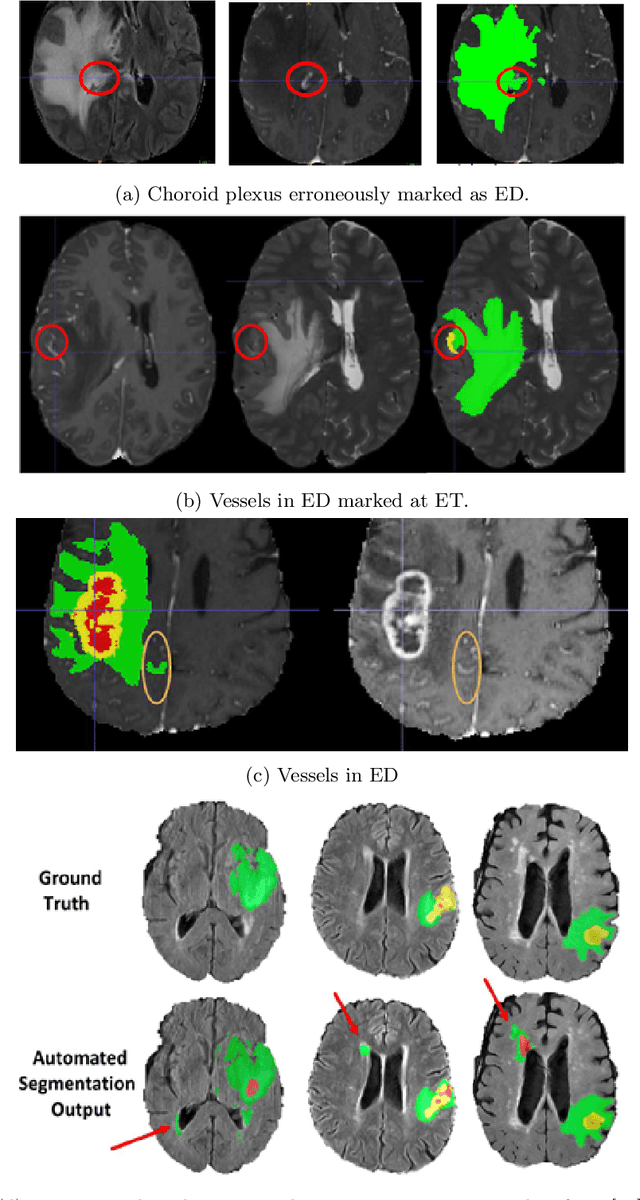
Abstract:The BraTS 2021 challenge celebrates its 10th anniversary and is jointly organized by the Radiological Society of North America (RSNA), the American Society of Neuroradiology (ASNR), and the Medical Image Computing and Computer Assisted Interventions (MICCAI) society. Since its inception, BraTS has been focusing on being a common benchmarking venue for brain glioma segmentation algorithms, with well-curated multi-institutional multi-parametric magnetic resonance imaging (mpMRI) data. Gliomas are the most common primary malignancies of the central nervous system, with varying degrees of aggressiveness and prognosis. The RSNA-ASNR-MICCAI BraTS 2021 challenge targets the evaluation of computational algorithms assessing the same tumor compartmentalization, as well as the underlying tumor's molecular characterization, in pre-operative baseline mpMRI data from 2,000 patients. Specifically, the two tasks that BraTS 2021 focuses on are: a) the segmentation of the histologically distinct brain tumor sub-regions, and b) the classification of the tumor's O[6]-methylguanine-DNA methyltransferase (MGMT) promoter methylation status. The performance evaluation of all participating algorithms in BraTS 2021 will be conducted through the Sage Bionetworks Synapse platform (Task 1) and Kaggle (Task 2), concluding in distributing to the top ranked participants monetary awards of $60,000 collectively.
A community-powered search of machine learning strategy space to find NMR property prediction models
Aug 13, 2020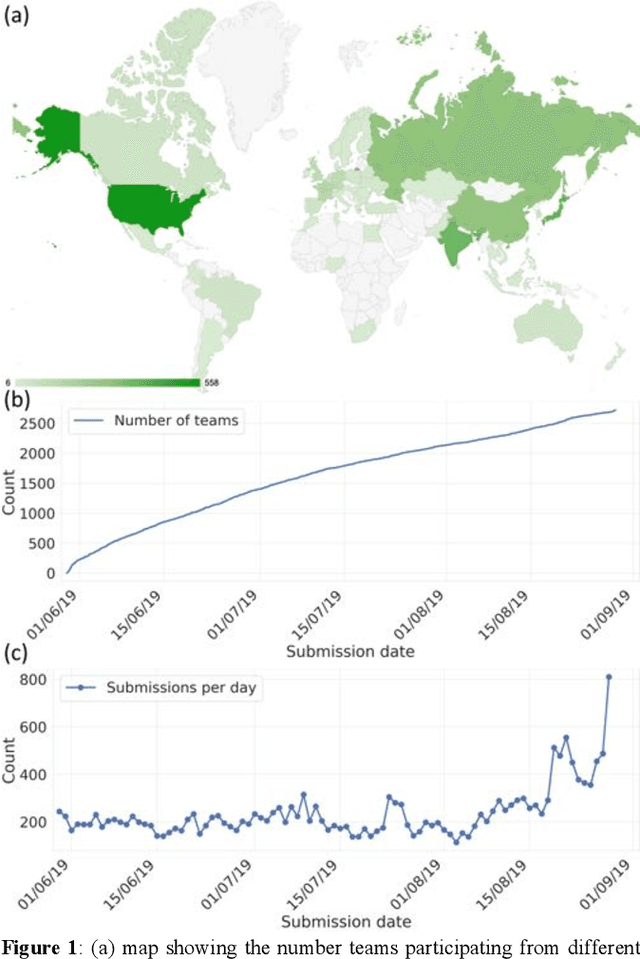
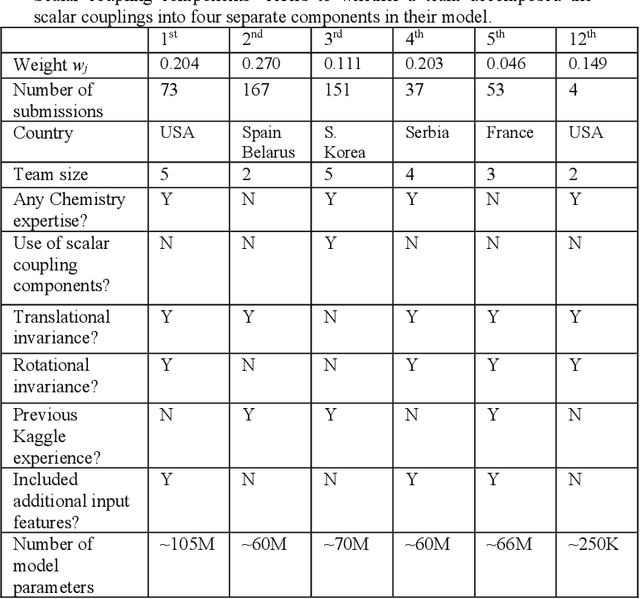
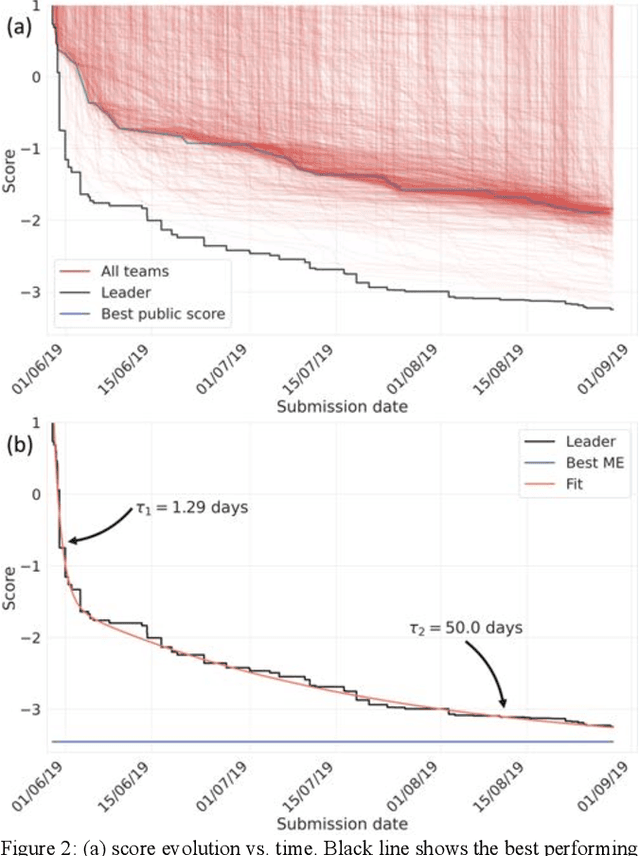
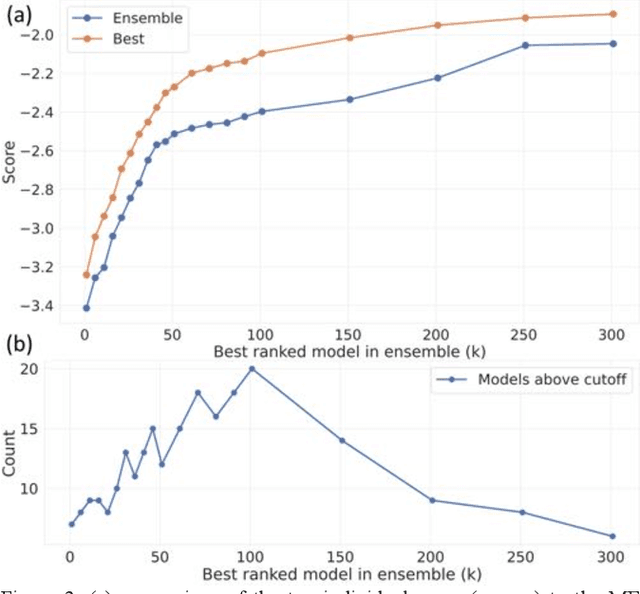
Abstract:The rise of machine learning (ML) has created an explosion in the potential strategies for using data to make scientific predictions. For physical scientists wishing to apply ML strategies to a particular domain, it can be difficult to assess in advance what strategy to adopt within a vast space of possibilities. Here we outline the results of an online community-powered effort to swarm search the space of ML strategies and develop algorithms for predicting atomic-pairwise nuclear magnetic resonance (NMR) properties in molecules. Using an open-source dataset, we worked with Kaggle to design and host a 3-month competition which received 47,800 ML model predictions from 2,700 teams in 84 countries. Within 3 weeks, the Kaggle community produced models with comparable accuracy to our best previously published "in-house" efforts. A meta-ensemble model constructed as a linear combination of the top predictions has a prediction accuracy which exceeds that of any individual model, 7-19x better than our previous state-of-the-art. The results highlight the potential of transformer architectures for predicting quantum mechanical (QM) molecular properties.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge