Uygar Sümbül
Learning Time-Invariant Representations for Individual Neurons from Population Dynamics
Nov 03, 2023



Abstract:Neurons can display highly variable dynamics. While such variability presumably supports the wide range of behaviors generated by the organism, their gene expressions are relatively stable in the adult brain. This suggests that neuronal activity is a combination of its time-invariant identity and the inputs the neuron receives from the rest of the circuit. Here, we propose a self-supervised learning based method to assign time-invariant representations to individual neurons based on permutation-, and population size-invariant summary of population recordings. We fit dynamical models to neuronal activity to learn a representation by considering the activity of both the individual and the neighboring population. Our self-supervised approach and use of implicit representations enable robust inference against imperfections such as partial overlap of neurons across sessions, trial-to-trial variability, and limited availability of molecular (transcriptomic) labels for downstream supervised tasks. We demonstrate our method on a public multimodal dataset of mouse cortical neuronal activity and transcriptomic labels. We report > 35% improvement in predicting the transcriptomic subclass identity and > 20% improvement in predicting class identity with respect to the state-of-the-art.
Biologically-plausible backpropagation through arbitrary timespans via local neuromodulators
Jun 02, 2022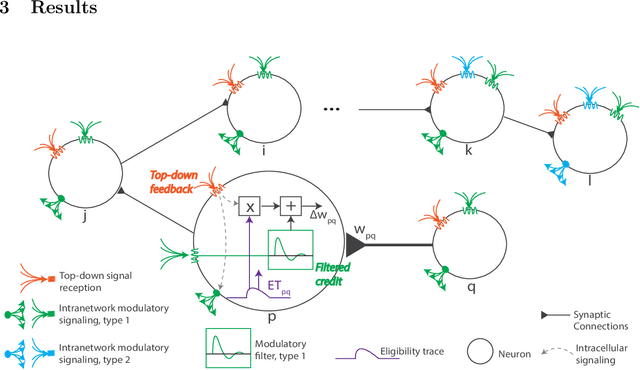
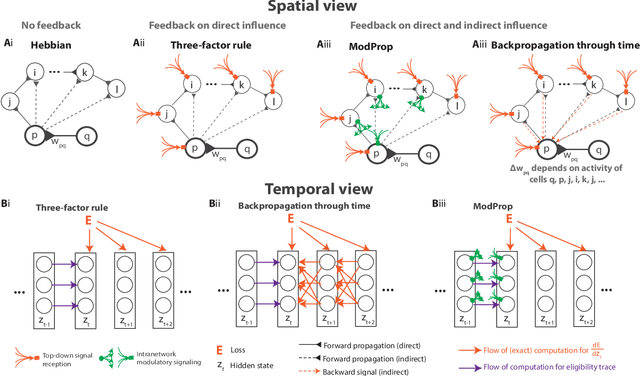
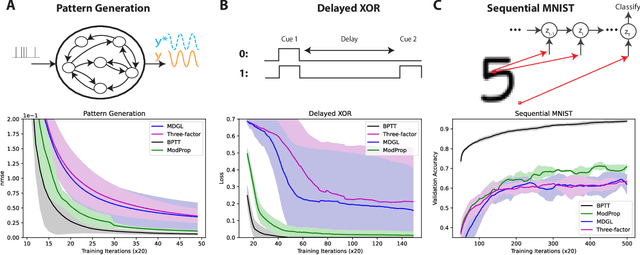
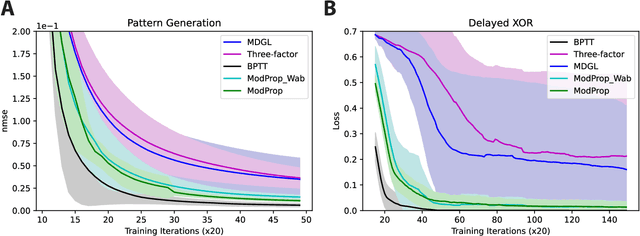
Abstract:The spectacular successes of recurrent neural network models where key parameters are adjusted via backpropagation-based gradient descent have inspired much thought as to how biological neuronal networks might solve the corresponding synaptic credit assignment problem. There is so far little agreement, however, as to how biological networks could implement the necessary backpropagation through time, given widely recognized constraints of biological synaptic network signaling architectures. Here, we propose that extra-synaptic diffusion of local neuromodulators such as neuropeptides may afford an effective mode of backpropagation lying within the bounds of biological plausibility. Going beyond existing temporal truncation-based gradient approximations, our approximate gradient-based update rule, ModProp, propagates credit information through arbitrary time steps. ModProp suggests that modulatory signals can act on receiving cells by convolving their eligibility traces via causal, time-invariant and synapse-type-specific filter taps. Our mathematical analysis of ModProp learning, together with simulation results on benchmark temporal tasks, demonstrate the advantage of ModProp over existing biologically-plausible temporal credit assignment rules. These results suggest a potential neuronal mechanism for signaling credit information related to recurrent interactions over a longer time horizon. Finally, we derive an in-silico implementation of ModProp that could serve as a low-complexity and causal alternative to backpropagation through time.
Joint Learning of Discrete and Continuous Variability with Coupled Autoencoding Agents
Jul 20, 2020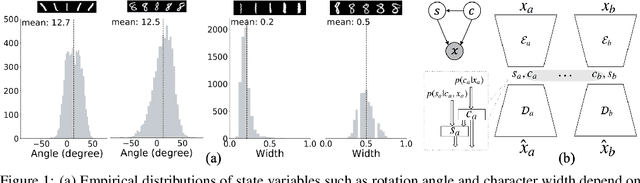
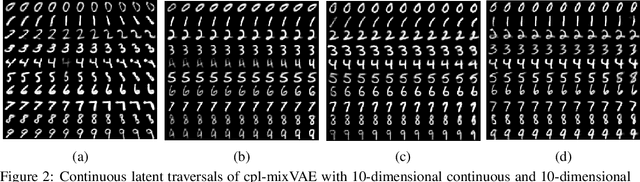
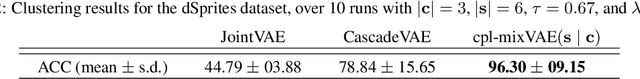
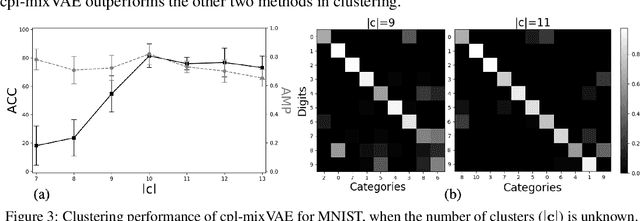
Abstract:Jointly identifying discrete and continuous factors of variability can help unravel complex phenomena. In neuroscience, a high-priority instance of this problem is the analysis of neuronal identity. Here, we study this problem in a variational framework by utilizing interacting autoencoding agents, designed to function in the absence of prior distribution over the discrete variable and reach collective decisions. We provide theoretical justification for our method and demonstrate improvements in terms of interpretability, stability, and accuracy over comparable approaches with experimental results on two benchmark datasets and a recent dataset of gene expression profiles of mouse cortical neurons. Furthermore, we demonstrate how our method can determine the neuronal cell types in an unsupervised setting, while identifying the genes implicated in regulating biologically relevant neuronal states.
A coupled autoencoder approach for multi-modal analysis of cell types
Nov 06, 2019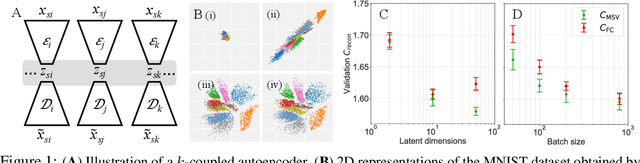
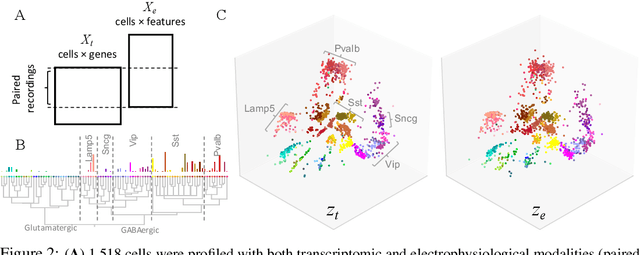
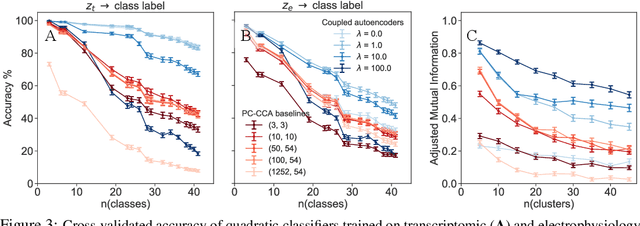
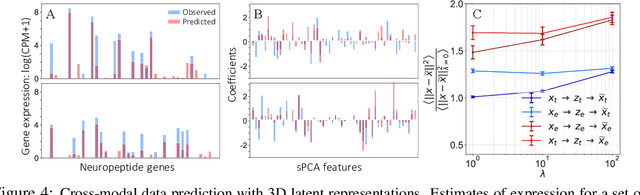
Abstract:Recent developments in high throughput profiling of individual neurons have spurred data driven exploration of the idea that there exist natural groupings of neurons referred to as cell types. The promise of this idea is that the immense complexity of brain circuits can be reduced, and effectively studied by means of interactions between cell types. While clustering of neuron populations based on a particular data modality can be used to define cell types, such definitions are often inconsistent across different characterization modalities. We pose this issue of cross-modal alignment as an optimization problem and develop an approach based on coupled training of autoencoders as a framework for such analyses. We apply this framework to a Patch-seq dataset consisting of transcriptomic and electrophysiological profiles for the same set of neurons to study consistency of representations across modalities, and evaluate cross-modal data prediction ability. We explore the problem where only a subset of neurons is characterized with more than one modality, and demonstrate that representations learned by coupled autoencoders can be used to identify types sampled only by a single modality.
Reconstructing neuronal anatomy from whole-brain images
Mar 17, 2019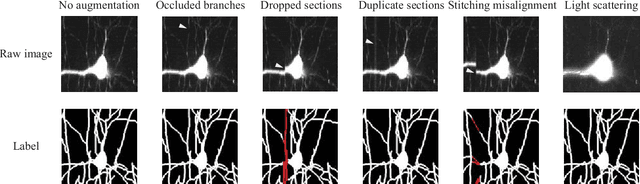
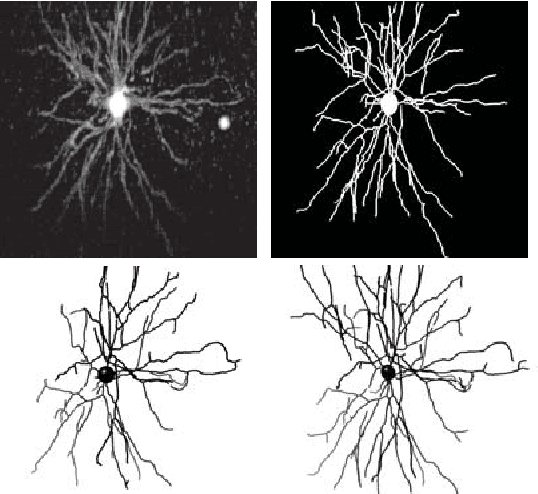
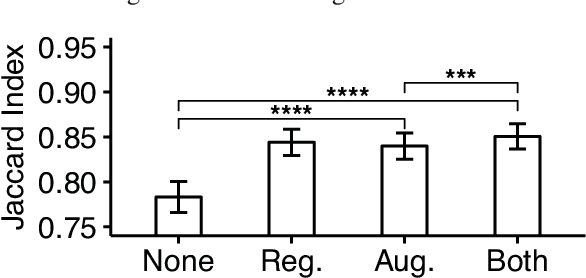
Abstract:Reconstructing multiple molecularly defined neurons from individual brains and across multiple brain regions can reveal organizational principles of the nervous system. However, high resolution imaging of the whole brain is a technically challenging and slow process. Recently, oblique light sheet microscopy has emerged as a rapid imaging method that can provide whole brain fluorescence microscopy at a voxel size of 0.4 by 0.4 by 2.5 cubic microns. On the other hand, complex image artifacts due to whole-brain coverage produce apparent discontinuities in neuronal arbors. Here, we present connectivity-preserving methods and data augmentation strategies for supervised learning of neuroanatomy from light microscopy using neural networks. We quantify the merit of our approach by implementing an end-to-end automated tracing pipeline. Lastly, we demonstrate a scalable, distributed implementation that can reconstruct the large datasets that sub-micron whole-brain images produce.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge