Kannan Umadevi Venkataraju
A streamable large-scale clinical EEG dataset for Deep Learning
Apr 13, 2022


Abstract:Deep Learning has revolutionized various fields, including Computer Vision, Natural Language Processing, as well as Biomedical research. Within the field of neuroscience, specifically in electrophysiological neuroimaging, researchers are starting to explore leveraging deep learning to make predictions on their data without extensive feature engineering. The availability of large-scale datasets is a crucial aspect of allowing the experimentation of Deep Learning models. We are publishing the first large-scale clinical EEG dataset that simplifies data access and management for Deep Learning. This dataset contains eyes-closed EEG data prepared from a collection of 1,574 juvenile participants from the Healthy Brain Network. We demonstrate a use case integrating this framework, and discuss why providing such neuroinformatics infrastructure to the community is critical for future scientific discoveries.
Reconstructing neuronal anatomy from whole-brain images
Mar 17, 2019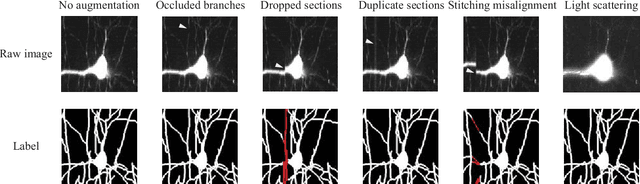
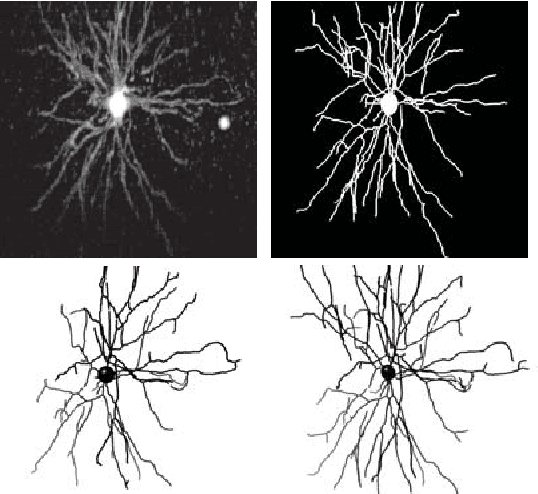
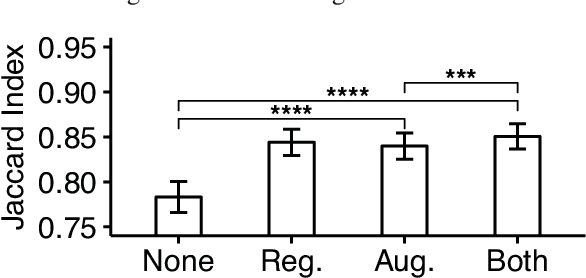
Abstract:Reconstructing multiple molecularly defined neurons from individual brains and across multiple brain regions can reveal organizational principles of the nervous system. However, high resolution imaging of the whole brain is a technically challenging and slow process. Recently, oblique light sheet microscopy has emerged as a rapid imaging method that can provide whole brain fluorescence microscopy at a voxel size of 0.4 by 0.4 by 2.5 cubic microns. On the other hand, complex image artifacts due to whole-brain coverage produce apparent discontinuities in neuronal arbors. Here, we present connectivity-preserving methods and data augmentation strategies for supervised learning of neuroanatomy from light microscopy using neural networks. We quantify the merit of our approach by implementing an end-to-end automated tracing pipeline. Lastly, we demonstrate a scalable, distributed implementation that can reconstruct the large datasets that sub-micron whole-brain images produce.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge