Sudarshan Regmi
AdaSCALE: Adaptive Scaling for OOD Detection
Mar 11, 2025Abstract:The ability of the deep learning model to recognize when a sample falls outside its learned distribution is critical for safe and reliable deployment. Recent state-of-the-art out-of-distribution (OOD) detection methods leverage activation shaping to improve the separation between in-distribution (ID) and OOD inputs. These approaches resort to sample-specific scaling but apply a static percentile threshold across all samples regardless of their nature, resulting in suboptimal ID-OOD separability. In this work, we propose \textbf{AdaSCALE}, an adaptive scaling procedure that dynamically adjusts the percentile threshold based on a sample's estimated OOD likelihood. This estimation leverages our key observation: OOD samples exhibit significantly more pronounced activation shifts at high-magnitude activations under minor perturbation compared to ID samples. AdaSCALE enables stronger scaling for likely ID samples and weaker scaling for likely OOD samples, yielding highly separable energy scores. Our approach achieves state-of-the-art OOD detection performance, outperforming the latest rival OptFS by 14.94 in near-OOD and 21.67 in far-OOD datasets in average FPR@95 metric on the ImageNet-1k benchmark across eight diverse architectures. The code is available at: https://github.com/sudarshanregmi/AdaSCALE/
Going Beyond Conventional OOD Detection
Nov 16, 2024Abstract:Out-of-distribution (OOD) detection is critical to ensure the safe deployment of deep learning models in critical applications. Deep learning models can often misidentify OOD samples as in-distribution (ID) samples. This vulnerability worsens in the presence of spurious correlation in the training set. Likewise, in fine-grained classification settings, detection of fine-grained OOD samples becomes inherently challenging due to their high similarity to ID samples. However, current research on OOD detection has largely ignored these challenging scenarios, focusing instead on relatively easier (conventional) cases. In this work, we present a unified Approach to Spurious, fine-grained, and Conventional OOD Detection (ASCOOD). First, we propose synthesizing virtual outliers from ID data by approximating the destruction of invariant features. We identify invariant features with the pixel attribution method using the model being learned. This approach eliminates the burden of curating external OOD datasets. Then, we simultaneously incentivize ID classification and predictive uncertainty towards the virtual outliers leveraging standardized feature representation. Our approach effectively mitigates the impact of spurious correlations and encourages capturing fine-grained attributes. Extensive experiments across six datasets demonstrate the merit of ASCOOD in spurious, fine-grained, and conventional settings. The code is available at: https://github.com/sudarshanregmi/ASCOOD/
Neural Network Pruning for Real-time Polyp Segmentation
Jun 22, 2023Abstract:Computer-assisted treatment has emerged as a viable application of medical imaging, owing to the efficacy of deep learning models. Real-time inference speed remains a key requirement for such applications to help medical personnel. Even though there generally exists a trade-off between performance and model size, impressive efforts have been made to retain near-original performance by compromising model size. Neural network pruning has emerged as an exciting area that aims to eliminate redundant parameters to make the inference faster. In this study, we show an application of neural network pruning in polyp segmentation. We compute the importance score of convolutional filters and remove the filters having the least scores, which to some value of pruning does not degrade the performance. For computing the importance score, we use the Taylor First Order (TaylorFO) approximation of the change in network output for the removal of certain filters. Specifically, we employ a gradient-normalized backpropagation for the computation of the importance score. Through experiments in the polyp datasets, we validate that our approach can significantly reduce the parameter count and FLOPs retaining similar performance.
T2FNorm: Extremely Simple Scaled Train-time Feature Normalization for OOD Detection
Jun 08, 2023



Abstract:Neural networks are notorious for being overconfident predictors, posing a significant challenge to their safe deployment in real-world applications. While feature normalization has garnered considerable attention within the deep learning literature, current train-time regularization methods for Out-of-Distribution(OOD) detection are yet to fully exploit this potential. Indeed, the naive incorporation of feature normalization within neural networks does not guarantee substantial improvement in OOD detection performance. In this work, we introduce T2FNorm, a novel approach to transforming features to hyperspherical space during training, while employing non-transformed space for OOD-scoring purposes. This method yields a surprising enhancement in OOD detection capabilities without compromising model accuracy in in-distribution(ID). Our investigation demonstrates that the proposed technique substantially diminishes the norm of the features of all samples, more so in the case of out-of-distribution samples, thereby addressing the prevalent concern of overconfidence in neural networks. The proposed method also significantly improves various post-hoc OOD detection methods.
CholecTriplet2022: Show me a tool and tell me the triplet -- an endoscopic vision challenge for surgical action triplet detection
Feb 13, 2023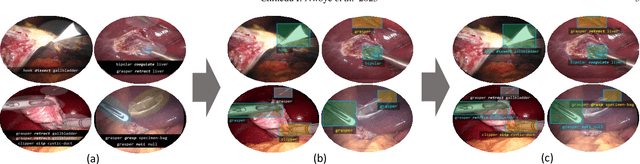
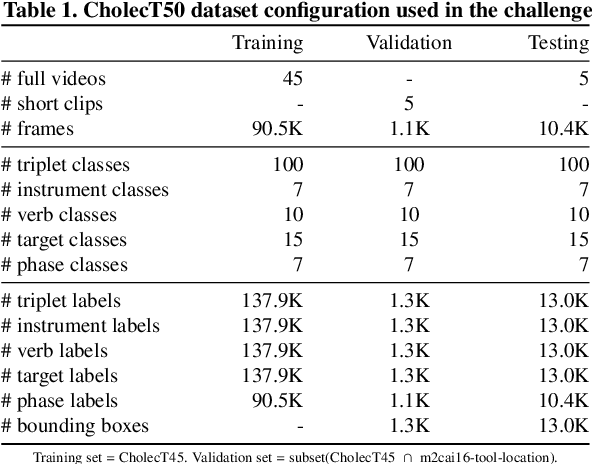
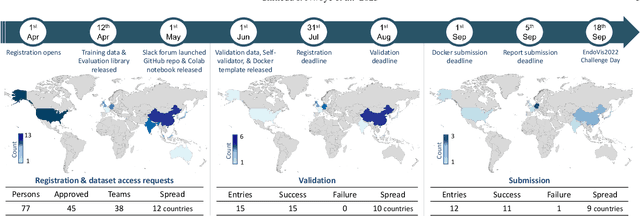
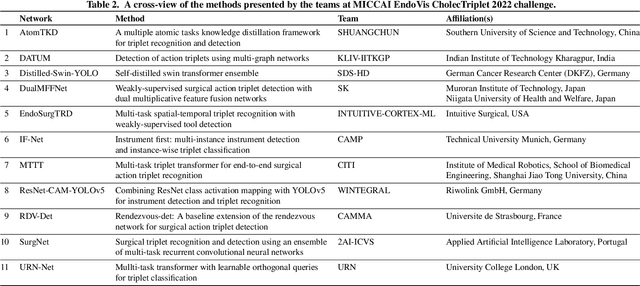
Abstract:Formalizing surgical activities as triplets of the used instruments, actions performed, and target anatomies is becoming a gold standard approach for surgical activity modeling. The benefit is that this formalization helps to obtain a more detailed understanding of tool-tissue interaction which can be used to develop better Artificial Intelligence assistance for image-guided surgery. Earlier efforts and the CholecTriplet challenge introduced in 2021 have put together techniques aimed at recognizing these triplets from surgical footage. Estimating also the spatial locations of the triplets would offer a more precise intraoperative context-aware decision support for computer-assisted intervention. This paper presents the CholecTriplet2022 challenge, which extends surgical action triplet modeling from recognition to detection. It includes weakly-supervised bounding box localization of every visible surgical instrument (or tool), as the key actors, and the modeling of each tool-activity in the form of <instrument, verb, target> triplet. The paper describes a baseline method and 10 new deep learning algorithms presented at the challenge to solve the task. It also provides thorough methodological comparisons of the methods, an in-depth analysis of the obtained results, their significance, and useful insights for future research directions and applications in surgery.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge