Srigokul Upadhyayula
Fourier-Based 3D Multistage Transformer for Aberration Correction in Multicellular Specimens
Mar 16, 2025Abstract:High-resolution tissue imaging is often compromised by sample-induced optical aberrations that degrade resolution and contrast. While wavefront sensor-based adaptive optics (AO) can measure these aberrations, such hardware solutions are typically complex, expensive to implement, and slow when serially mapping spatially varying aberrations across large fields of view. Here, we introduce AOViFT (Adaptive Optical Vision Fourier Transformer) -- a machine learning-based aberration sensing framework built around a 3D multistage Vision Transformer that operates on Fourier domain embeddings. AOViFT infers aberrations and restores diffraction-limited performance in puncta-labeled specimens with substantially reduced computational cost, training time, and memory footprint compared to conventional architectures or real-space networks. We validated AOViFT on live gene-edited zebrafish embryos, demonstrating its ability to correct spatially varying aberrations using either a deformable mirror or post-acquisition deconvolution. By eliminating the need for the guide star and wavefront sensing hardware and simplifying the experimental workflow, AOViFT lowers technical barriers for high-resolution volumetric microscopy across diverse biological samples.
How to Build the Virtual Cell with Artificial Intelligence: Priorities and Opportunities
Sep 18, 2024

Abstract:The cell is arguably the smallest unit of life and is central to understanding biology. Accurate modeling of cells is important for this understanding as well as for determining the root causes of disease. Recent advances in artificial intelligence (AI), combined with the ability to generate large-scale experimental data, present novel opportunities to model cells. Here we propose a vision of AI-powered Virtual Cells, where robust representations of cells and cellular systems under different conditions are directly learned from growing biological data across measurements and scales. We discuss desired capabilities of AI Virtual Cells, including generating universal representations of biological entities across scales, and facilitating interpretable in silico experiments to predict and understand their behavior using Virtual Instruments. We further address the challenges, opportunities and requirements to realize this vision including data needs, evaluation strategies, and community standards and engagement to ensure biological accuracy and broad utility. We envision a future where AI Virtual Cells help identify new drug targets, predict cellular responses to perturbations, as well as scale hypothesis exploration. With open science collaborations across the biomedical ecosystem that includes academia, philanthropy, and the biopharma and AI industries, a comprehensive predictive understanding of cell mechanisms and interactions is within reach.
Image-to-Image Regression with Distribution-Free Uncertainty Quantification and Applications in Imaging
Feb 10, 2022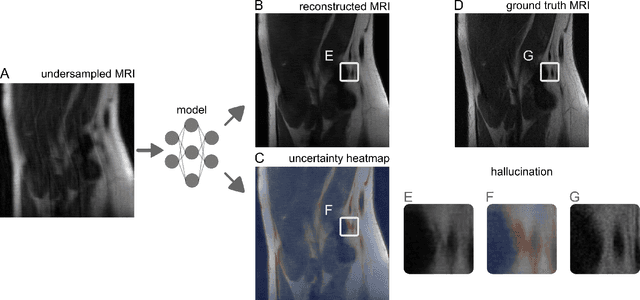
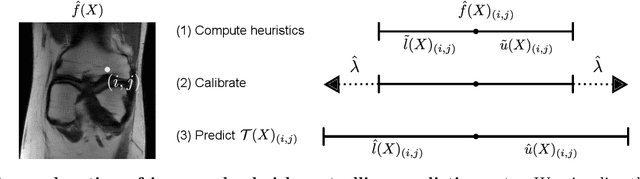
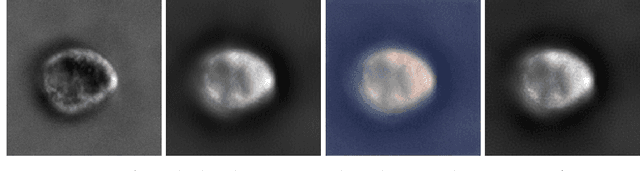
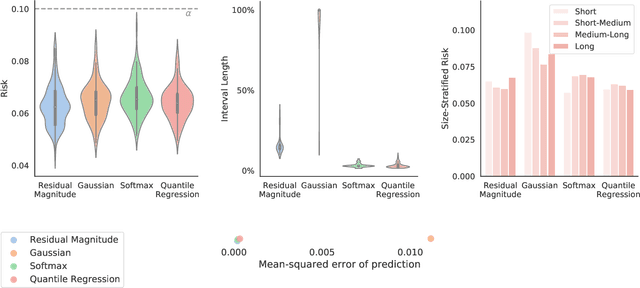
Abstract:Image-to-image regression is an important learning task, used frequently in biological imaging. Current algorithms, however, do not generally offer statistical guarantees that protect against a model's mistakes and hallucinations. To address this, we develop uncertainty quantification techniques with rigorous statistical guarantees for image-to-image regression problems. In particular, we show how to derive uncertainty intervals around each pixel that are guaranteed to contain the true value with a user-specified confidence probability. Our methods work in conjunction with any base machine learning model, such as a neural network, and endow it with formal mathematical guarantees -- regardless of the true unknown data distribution or choice of model. Furthermore, they are simple to implement and computationally inexpensive. We evaluate our procedure on three image-to-image regression tasks: quantitative phase microscopy, accelerated magnetic resonance imaging, and super-resolution transmission electron microscopy of a Drosophila melanogaster brain.
Adaptive wavelet distillation from neural networks through interpretations
Jul 19, 2021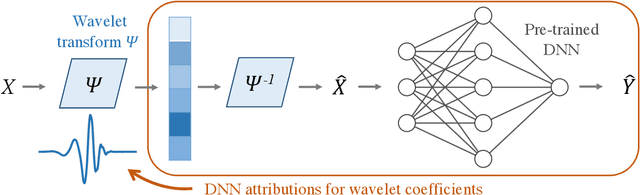
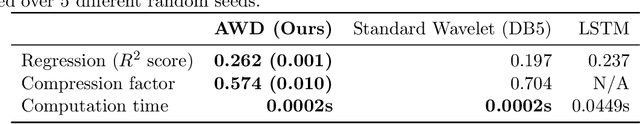
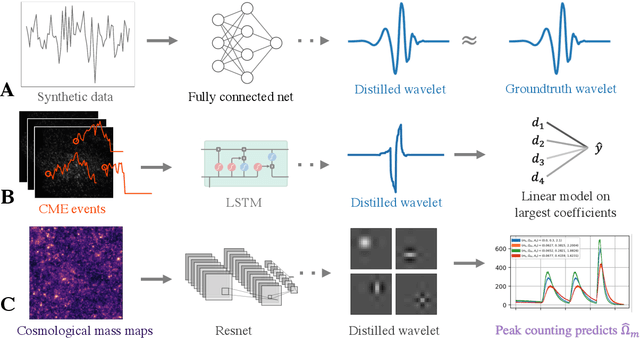

Abstract:Recent deep-learning models have achieved impressive prediction performance, but often sacrifice interpretability and computational efficiency. Interpretability is crucial in many disciplines, such as science and medicine, where models must be carefully vetted or where interpretation is the goal itself. Moreover, interpretable models are concise and often yield computational efficiency. Here, we propose adaptive wavelet distillation (AWD), a method which aims to distill information from a trained neural network into a wavelet transform. Specifically, AWD penalizes feature attributions of a neural network in the wavelet domain to learn an effective multi-resolution wavelet transform. The resulting model is highly predictive, concise, computationally efficient, and has properties (such as a multi-scale structure) which make it easy to interpret. In close collaboration with domain experts, we showcase how AWD addresses challenges in two real-world settings: cosmological parameter inference and molecular-partner prediction. In both cases, AWD yields a scientifically interpretable and concise model which gives predictive performance better than state-of-the-art neural networks. Moreover, AWD identifies predictive features that are scientifically meaningful in the context of respective domains. All code and models are released in a full-fledged package available on Github (https://github.com/Yu-Group/adaptive-wavelets).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge