Cyna Shirazinejad
Fourier-Based 3D Multistage Transformer for Aberration Correction in Multicellular Specimens
Mar 16, 2025Abstract:High-resolution tissue imaging is often compromised by sample-induced optical aberrations that degrade resolution and contrast. While wavefront sensor-based adaptive optics (AO) can measure these aberrations, such hardware solutions are typically complex, expensive to implement, and slow when serially mapping spatially varying aberrations across large fields of view. Here, we introduce AOViFT (Adaptive Optical Vision Fourier Transformer) -- a machine learning-based aberration sensing framework built around a 3D multistage Vision Transformer that operates on Fourier domain embeddings. AOViFT infers aberrations and restores diffraction-limited performance in puncta-labeled specimens with substantially reduced computational cost, training time, and memory footprint compared to conventional architectures or real-space networks. We validated AOViFT on live gene-edited zebrafish embryos, demonstrating its ability to correct spatially varying aberrations using either a deformable mirror or post-acquisition deconvolution. By eliminating the need for the guide star and wavefront sensing hardware and simplifying the experimental workflow, AOViFT lowers technical barriers for high-resolution volumetric microscopy across diverse biological samples.
ProDyn0: Inferring calponin homology domain stretching behavior using graph neural networks
Oct 22, 2019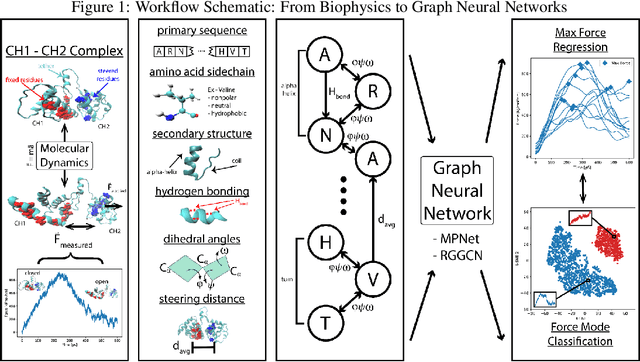
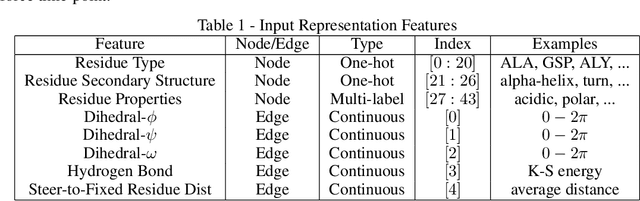
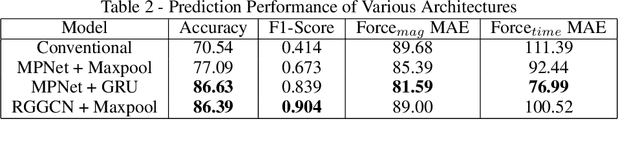
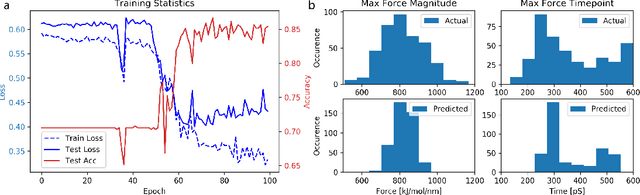
Abstract:Graph neural networks are a quickly emerging field for non-Euclidean data that leverage the inherent graphical structure to predict node, edge, and global-level properties of a system. Protein properties can not easily be understood as a simple sum of their parts (i.e. amino acids), therefore, understanding their dynamical properties in the context of graphs is attractive for revealing how perturbations to their structure can affect their global function. To tackle this problem, we generate a database of 2020 mutated calponin homology (CH) domains undergoing large-scale separation in molecular dynamics. To predict the mechanosensitive force response, we develop neural message passing networks and residual gated graph convnets which predict the protein dependent force separation at 86.63 percent, 81.59 kJ/mol/nm MAE, 76.99 psec MAE for force mode classification, max force magnitude, max force time respectively-- significantly better than non-graph-based deep learning techniques. Towards uniting geometric learning techniques and biophysical observables, we premiere our simulation database as a benchmark dataset for further development/evaluation of graph neural network architectures.
* 8 pages, 2 figures, 2 tables
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge