Shamira Perera
3D Structural Phenotype of the Optic Nerve Head at the Intersection of Glaucoma and Myopia - A Key to Improving Glaucoma Diagnosis in Myopic Populations
Mar 24, 2025Abstract:Purpose: To characterize the 3D structural phenotypes of the optic nerve head (ONH) in patients with glaucoma, high myopia, and concurrent high myopia and glaucoma, and to evaluate their variations across these conditions. Participants: A total of 685 optical coherence tomography (OCT) scans from 754 subjects of Singapore-Chinese ethnicity, including 256 healthy (H), 94 highly myopic (HM), 227 glaucomatous (G), and 108 highly myopic with glaucoma (HMG) cases. Methods: We segmented the retinal and connective tissues from OCT volumes and their boundary edges were converted into 3D point clouds. To classify the 3D point clouds into four ONH conditions, i.e., H, HM, G, and HMG, a specialized ensemble network was developed, consisting of an encoder to transform high-dimensional input data into a compressed latent vector, a decoder to reconstruct point clouds from the latent vector, and a classifier to categorize the point clouds into the four ONH conditions. Results: The classification network achieved high accuracy, distinguishing H, HM, G, and HMG classes with a micro-average AUC of 0.92 $\pm$ 0.03 on an independent test set. The decoder effectively reconstructed point clouds, achieving a Chamfer loss of 0.013 $\pm$ 0.002. Dimensionality reduction clustered ONHs into four distinct groups, revealing structural variations such as changes in retinal and connective tissue thickness, tilting and stretching of the disc and scleral canal opening, and alterations in optic cup morphology, including shallow or deep excavation, across the four conditions. Conclusions: This study demonstrated that ONHs exhibit distinct structural signatures across H, HM, G, and HMG conditions. The findings further indicate that ONH morphology provides sufficient information for classification into distinct clusters, with principal components capturing unique structural patterns within each group.
Introducing the Biomechanics-Function Relationship in Glaucoma: Improved Visual Field Loss Predictions from intraocular pressure-induced Neural Tissue Strains
Jun 21, 2024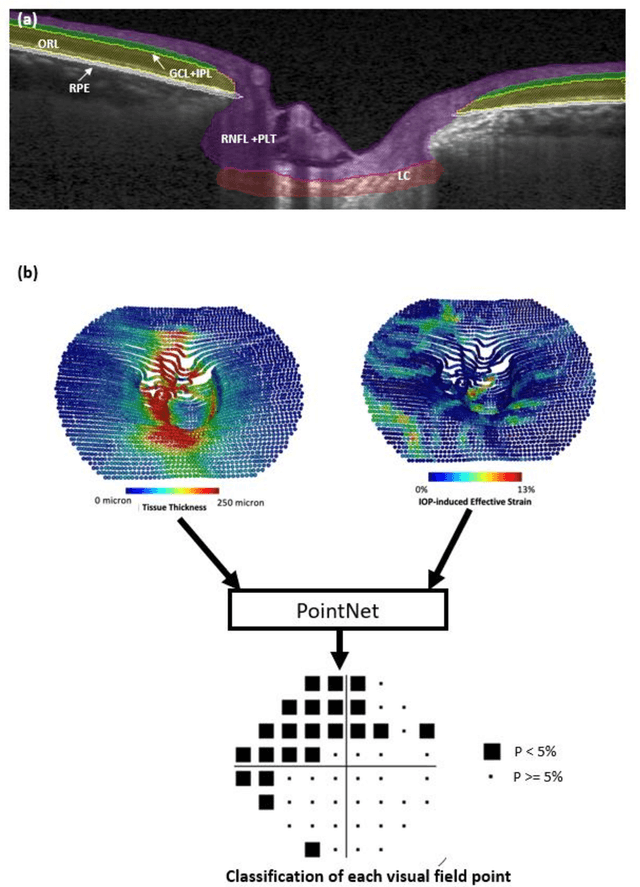

Abstract:Objective. (1) To assess whether neural tissue structure and biomechanics could predict functional loss in glaucoma; (2) To evaluate the importance of biomechanics in making such predictions. Design, Setting and Participants. We recruited 238 glaucoma subjects. For one eye of each subject, we imaged the optic nerve head (ONH) using spectral-domain OCT under the following conditions: (1) primary gaze and (2) primary gaze with acute IOP elevation. Main Outcomes: We utilized automatic segmentation of optic nerve head (ONH) tissues and digital volume correlation (DVC) analysis to compute intraocular pressure (IOP)-induced neural tissue strains. A robust geometric deep learning approach, known as Point-Net, was employed to predict the full Humphrey 24-2 pattern standard deviation (PSD) maps from ONH structural and biomechanical information. For each point in each PSD map, we predicted whether it exhibited no defect or a PSD value of less than 5%. Predictive performance was evaluated using 5-fold cross-validation and the F1-score. We compared the model's performance with and without the inclusion of IOP-induced strains to assess the impact of biomechanics on prediction accuracy. Results: Integrating biomechanical (IOP-induced neural tissue strains) and structural (tissue morphology and neural tissues thickness) information yielded a significantly better predictive model (F1-score: 0.76+-0.02) across validation subjects, as opposed to relying only on structural information, which resulted in a significantly lower F1-score of 0.71+-0.02 (p < 0.05). Conclusion: Our study has shown that the integration of biomechanical data can significantly improve the accuracy of visual field loss predictions. This highlights the importance of the biomechanics-function relationship in glaucoma, and suggests that biomechanics may serve as a crucial indicator for the development and progression of glaucoma.
The Three-Dimensional Structural Configuration of the Central Retinal Vessel Trunk and Branches as a Glaucoma Biomarker
Nov 09, 2021
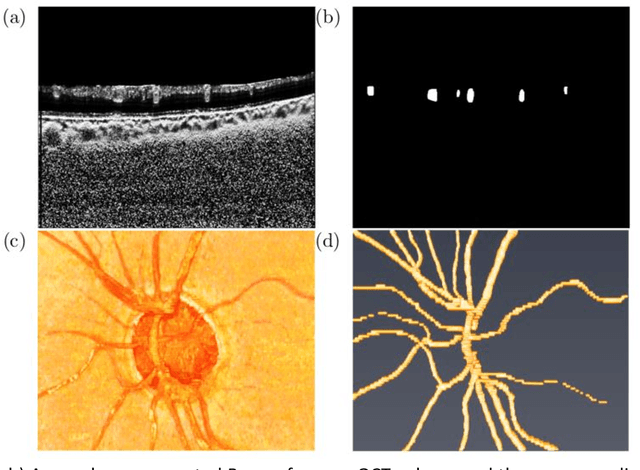
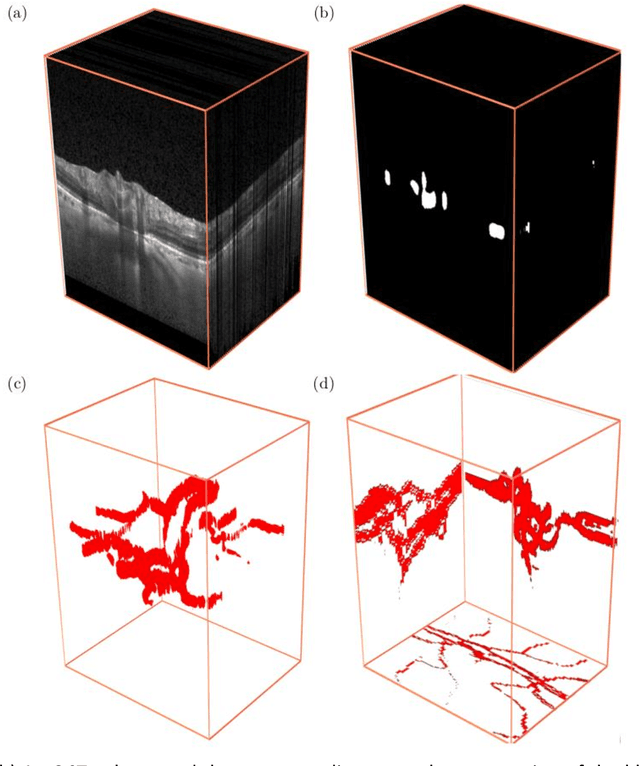

Abstract:Purpose: To assess whether the three-dimensional (3D) structural configuration of the central retinal vessel trunk and its branches (CRVT&B) could be used as a diagnostic marker for glaucoma. Method: We trained a deep learning network to automatically segment the CRVT&B from the B-scans of the optical coherence tomography (OCT) volume of the optic nerve head (ONH). Subsequently, two different approaches were used for glaucoma diagnosis using the structural configuration of the CRVT&B as extracted from the OCT volumes. In the first approach, we aimed to provide a diagnosis using only 3D CNN and the 3D structure of the CRVT&B. For the second approach, we projected the 3D structure of the CRVT&B orthographically onto three planes to obtain 2D images, and then a 2D CNN was used for diagnosis. The segmentation accuracy was evaluated using the Dice coefficient, whereas the diagnostic accuracy was assessed using the area under the receiver operating characteristic curves (AUC). The diagnostic performance of the CRVT&B was also compared with that of retinal nerve fiber layer (RNFL) thickness. Results: Our segmentation network was able to efficiently segment retinal blood vessels from OCT scans. On a test set, we achieved a Dice coefficient of 0.81\pm0.07. The 3D and 2D diagnostic networks were able to differentiate glaucoma from non-glaucoma subjects with accuracies of 82.7% and 83.3%, respectively. The corresponding AUCs for CRVT&B were 0.89 and 0.90, higher than those obtained with RNFL thickness alone. Conclusions: Our work demonstrated that the diagnostic power of the CRVT&B is superior to that of a gold-standard glaucoma parameter, i.e., RNFL thickness. Our work also suggested that the major retinal blood vessels form a skeleton -- the configuration of which may be representative of major ONH structural changes as typically observed with the development and progression of glaucoma.
Describing the Structural Phenotype of the Glaucomatous Optic Nerve Head Using Artificial Intelligence
Dec 17, 2020
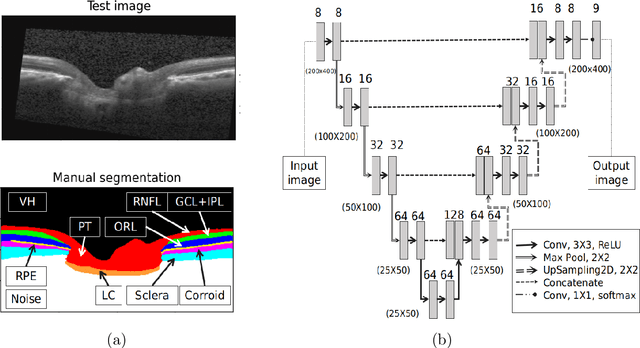
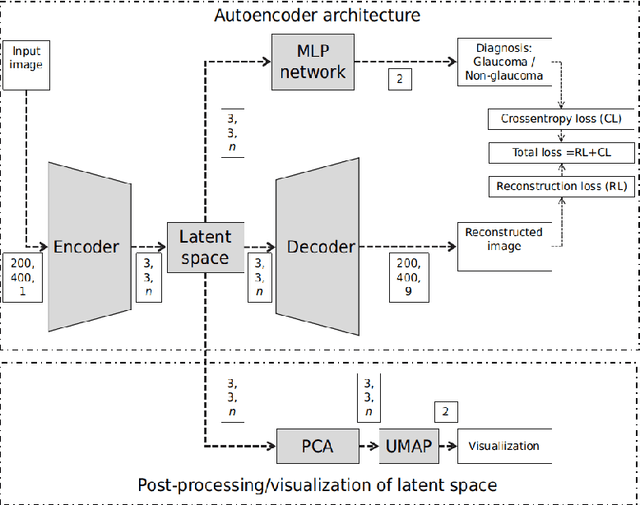

Abstract:The optic nerve head (ONH) typically experiences complex neural- and connective-tissue structural changes with the development and progression of glaucoma, and monitoring these changes could be critical for improved diagnosis and prognosis in the glaucoma clinic. The gold-standard technique to assess structural changes of the ONH clinically is optical coherence tomography (OCT). However, OCT is limited to the measurement of a few hand-engineered parameters, such as the thickness of the retinal nerve fiber layer (RNFL), and has not yet been qualified as a stand-alone device for glaucoma diagnosis and prognosis applications. We argue this is because the vast amount of information available in a 3D OCT scan of the ONH has not been fully exploited. In this study we propose a deep learning approach that can: \textbf{(1)} fully exploit information from an OCT scan of the ONH; \textbf{(2)} describe the structural phenotype of the glaucomatous ONH; and that can \textbf{(3)} be used as a robust glaucoma diagnosis tool. Specifically, the structural features identified by our algorithm were found to be related to clinical observations of glaucoma. The diagnostic accuracy from these structural features was $92.0 \pm 2.3 \%$ with a sensitivity of $90.0 \pm 2.4 \% $ (at $95 \%$ specificity). By changing their magnitudes in steps, we were able to reveal how the morphology of the ONH changes as one transitions from a `non-glaucoma' to a `glaucoma' condition. We believe our work may have strong clinical implication for our understanding of glaucoma pathogenesis, and could be improved in the future to also predict future loss of vision.
Towards Label-Free 3D Segmentation of Optical Coherence Tomography Images of the Optic Nerve Head Using Deep Learning
Feb 22, 2020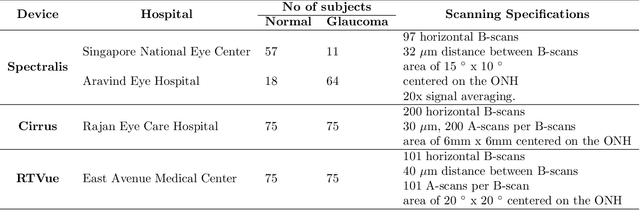
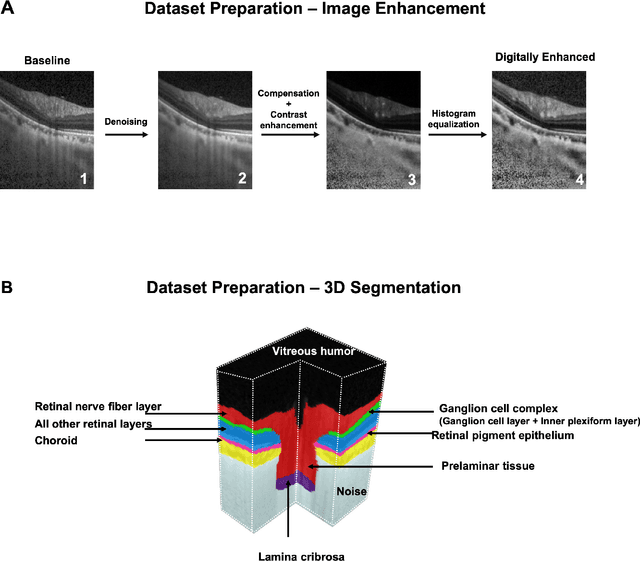
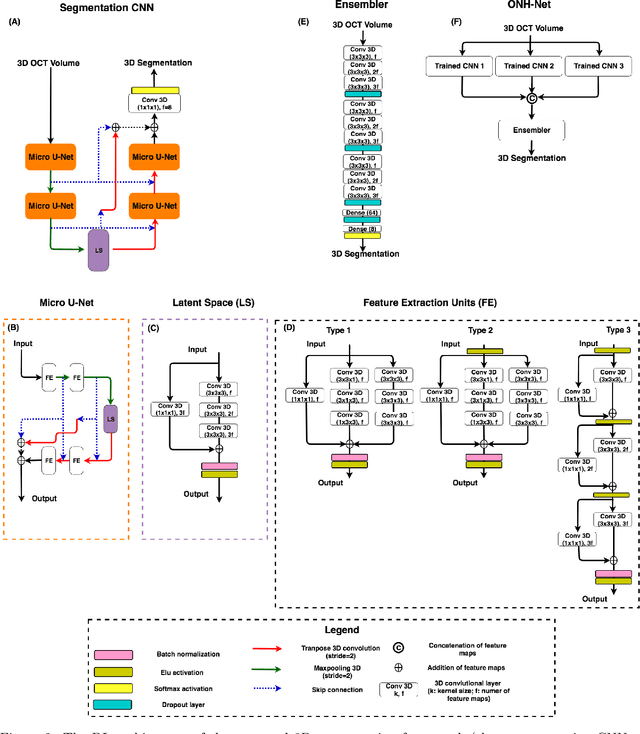
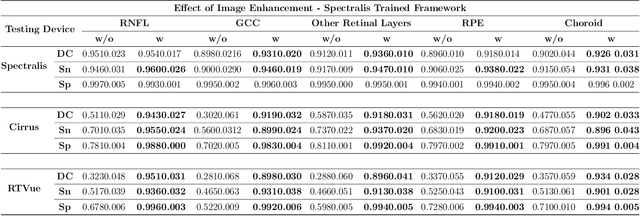
Abstract:Since the introduction of optical coherence tomography (OCT), it has been possible to study the complex 3D morphological changes of the optic nerve head (ONH) tissues that occur along with the progression of glaucoma. Although several deep learning (DL) techniques have been recently proposed for the automated extraction (segmentation) and quantification of these morphological changes, the device specific nature and the difficulty in preparing manual segmentations (training data) limit their clinical adoption. With several new manufacturers and next-generation OCT devices entering the market, the complexity in deploying DL algorithms clinically is only increasing. To address this, we propose a DL based 3D segmentation framework that is easily translatable across OCT devices in a label-free manner (i.e. without the need to manually re-segment data for each device). Specifically, we developed 2 sets of DL networks. The first (referred to as the enhancer) was able to enhance OCT image quality from 3 OCT devices, and harmonized image-characteristics across these devices. The second performed 3D segmentation of 6 important ONH tissue layers. We found that the use of the enhancer was critical for our segmentation network to achieve device independency. In other words, our 3D segmentation network trained on any of 3 devices successfully segmented ONH tissue layers from the other two devices with high performance (Dice coefficients > 0.92). With such an approach, we could automatically segment images from new OCT devices without ever needing manual segmentation data from such devices.
DeshadowGAN: A Deep Learning Approach to Remove Shadows from Optical Coherence Tomography Images
Oct 07, 2019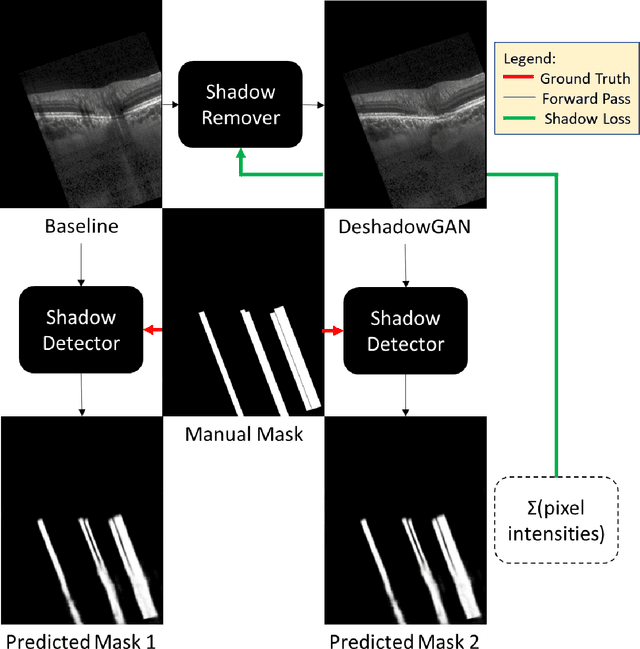
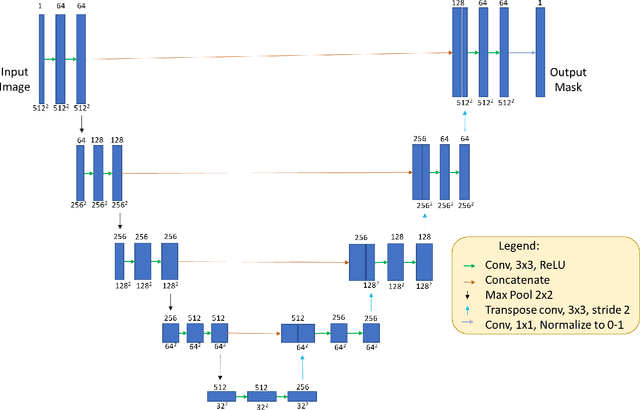
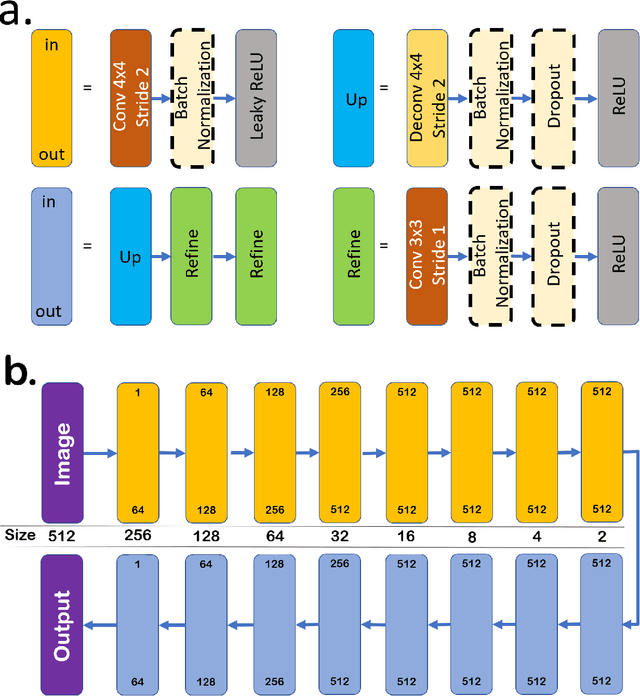
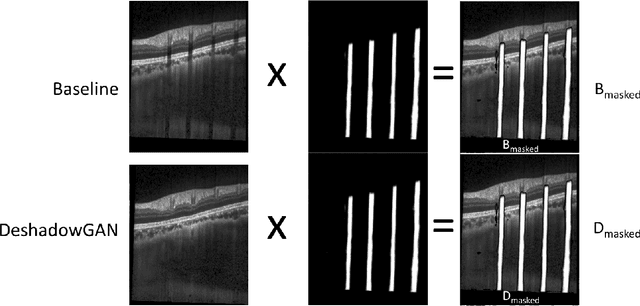
Abstract:Purpose: To remove retinal shadows from optical coherence tomography (OCT) images of the optic nerve head(ONH). Methods:2328 OCT images acquired through the center of the ONH using a Spectralis OCT machine for both eyes of 13 subjects were used to train a generative adversarial network (GAN) using a custom loss function. Image quality was assessed qualitatively (for artifacts) and quantitatively using the intralayer contrast: a measure of shadow visibility ranging from 0 (shadow-free) to 1 (strong shadow) and compared to compensated images. This was computed in the Retinal Nerve Fiber Layer (RNFL), the Inner Plexiform Layer (IPL), the Photoreceptor layer (PR) and the Retinal Pigment Epithelium (RPE) layers. Results: Output images had improved intralayer contrast in all ONH tissue layers. On average the intralayer contrast decreased by 33.7$\pm$6.81%, 28.8$\pm$10.4%, 35.9$\pm$13.0%, and43.0$\pm$19.5%for the RNFL, IPL, PR, and RPE layers respectively, indicating successful shadow removal across all depths. This compared to 70.3$\pm$22.7%, 33.9$\pm$11.5%, 47.0$\pm$11.2%, 26.7$\pm$19.0%for compensation. Output images were also free from artifacts commonly observed with compensation. Conclusions: DeshadowGAN significantly corrected blood vessel shadows in OCT images of the ONH. Our algorithm may be considered as a pre-processing step to improve the performance of a wide range of algorithms including those currently being used for OCT image segmentation, denoising, and classification. Translational Relevance: DeshadowGAN could be integrated to existing OCT devices to improve the diagnosis and prognosis of ocular pathologies.
A Deep Learning Approach to Denoise Optical Coherence Tomography Images of the Optic Nerve Head
Sep 27, 2018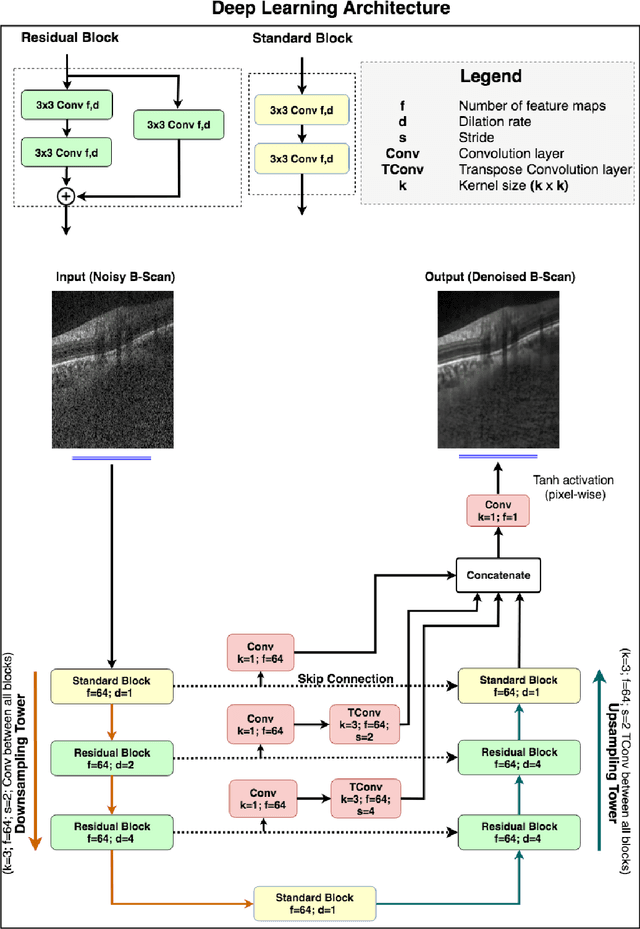
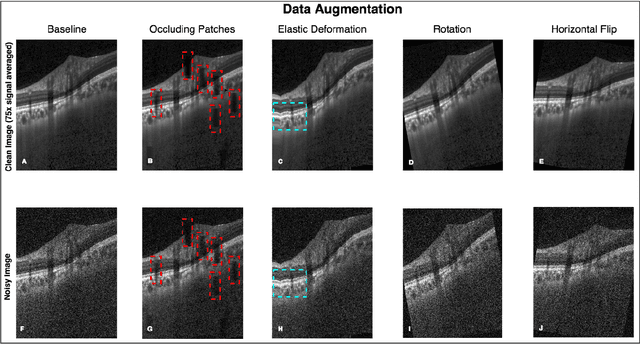
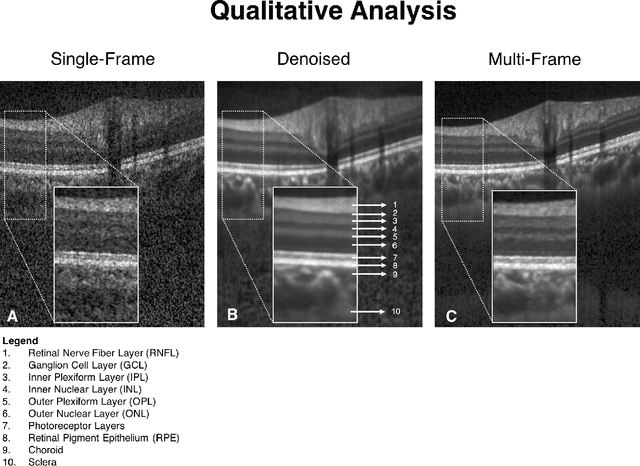
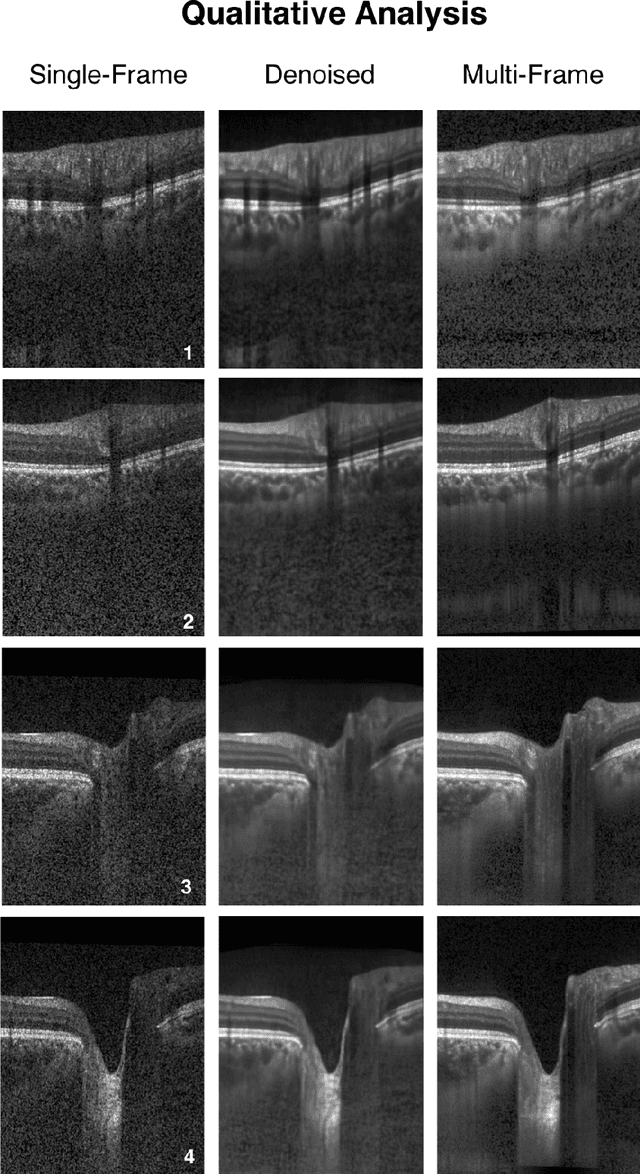
Abstract:Purpose: To develop a deep learning approach to de-noise optical coherence tomography (OCT) B-scans of the optic nerve head (ONH). Methods: Volume scans consisting of 97 horizontal B-scans were acquired through the center of the ONH using a commercial OCT device (Spectralis) for both eyes of 20 subjects. For each eye, single-frame (without signal averaging), and multi-frame (75x signal averaging) volume scans were obtained. A custom deep learning network was then designed and trained with 2,328 "clean B-scans" (multi-frame B-scans), and their corresponding "noisy B-scans" (clean B-scans + gaussian noise) to de-noise the single-frame B-scans. The performance of the de-noising algorithm was assessed qualitatively, and quantitatively on 1,552 B-scans using the signal to noise ratio (SNR), contrast to noise ratio (CNR), and mean structural similarity index metrics (MSSIM). Results: The proposed algorithm successfully denoised unseen single-frame OCT B-scans. The denoised B-scans were qualitatively similar to their corresponding multi-frame B-scans, with enhanced visibility of the ONH tissues. The mean SNR increased from $4.02 \pm 0.68$ dB (single-frame) to $8.14 \pm 1.03$ dB (denoised). For all the ONH tissues, the mean CNR increased from $3.50 \pm 0.56$ (single-frame) to $7.63 \pm 1.81$ (denoised). The MSSIM increased from $0.13 \pm 0.02$ (single frame) to $0.65 \pm 0.03$ (denoised) when compared with the corresponding multi-frame B-scans. Conclusions: Our deep learning algorithm can denoise a single-frame OCT B-scan of the ONH in under 20 ms, thus offering a framework to obtain superior quality OCT B-scans with reduced scanning times and minimal patient discomfort.
DRUNET: A Dilated-Residual U-Net Deep Learning Network to Digitally Stain Optic Nerve Head Tissues in Optical Coherence Tomography Images
Mar 01, 2018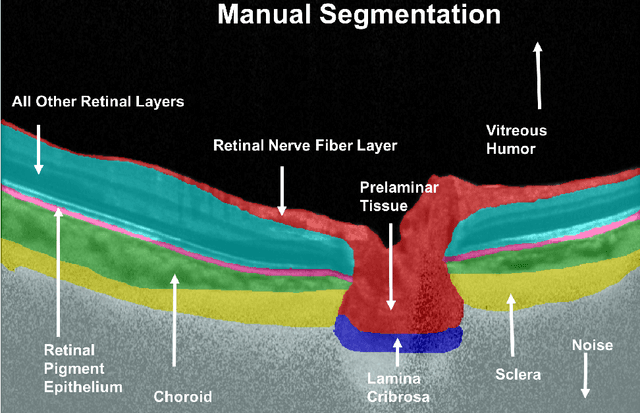
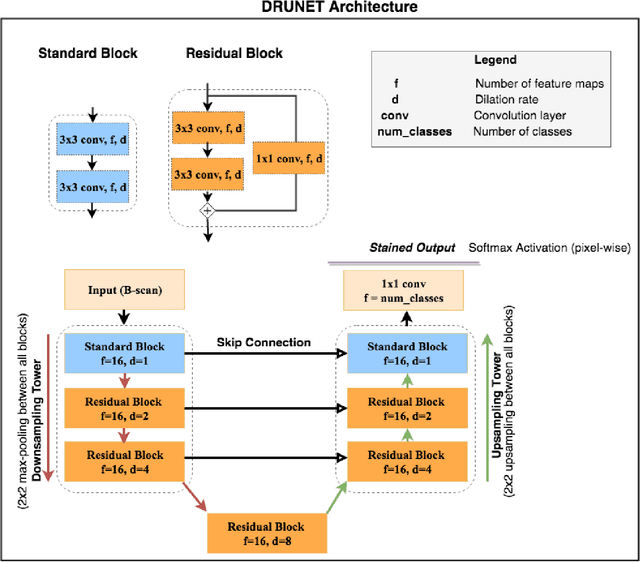
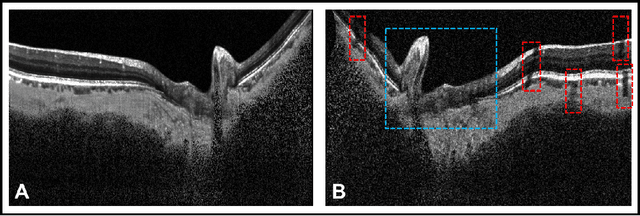
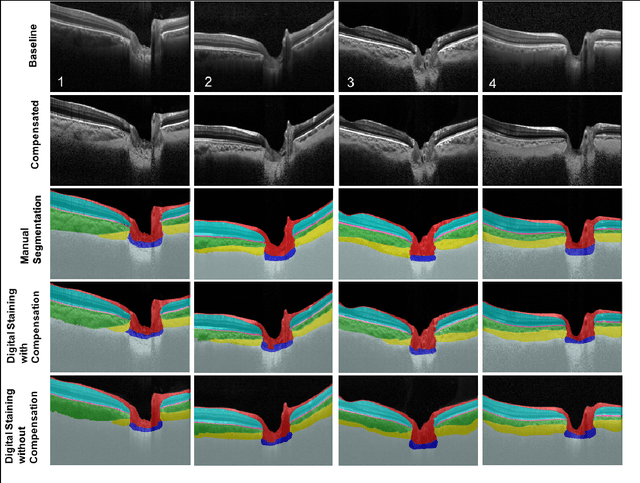
Abstract:Given that the neural and connective tissues of the optic nerve head (ONH) exhibit complex morphological changes with the development and progression of glaucoma, their simultaneous isolation from optical coherence tomography (OCT) images may be of great interest for the clinical diagnosis and management of this pathology. A deep learning algorithm was designed and trained to digitally stain (i.e. highlight) 6 ONH tissue layers by capturing both the local (tissue texture) and contextual information (spatial arrangement of tissues). The overall dice coefficient (mean of all tissues) was $0.91 \pm 0.05$ when assessed against manual segmentations performed by an expert observer. We offer here a robust segmentation framework that could be extended for the automated parametric study of the ONH tissues.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge