Satish K. Panda
3D Structural Analysis of the Optic Nerve Head to Robustly Discriminate Between Papilledema and Optic Disc Drusen
Dec 18, 2021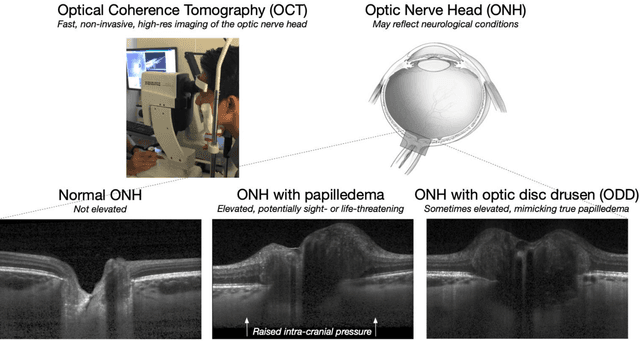
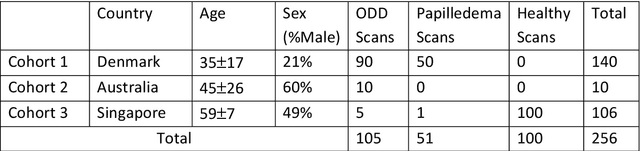
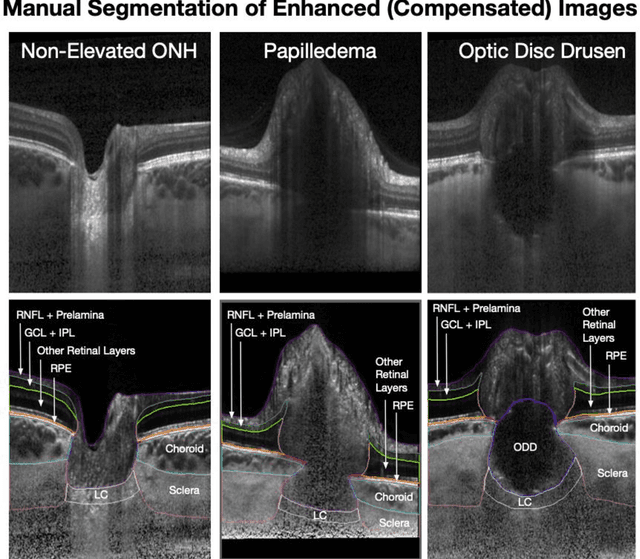
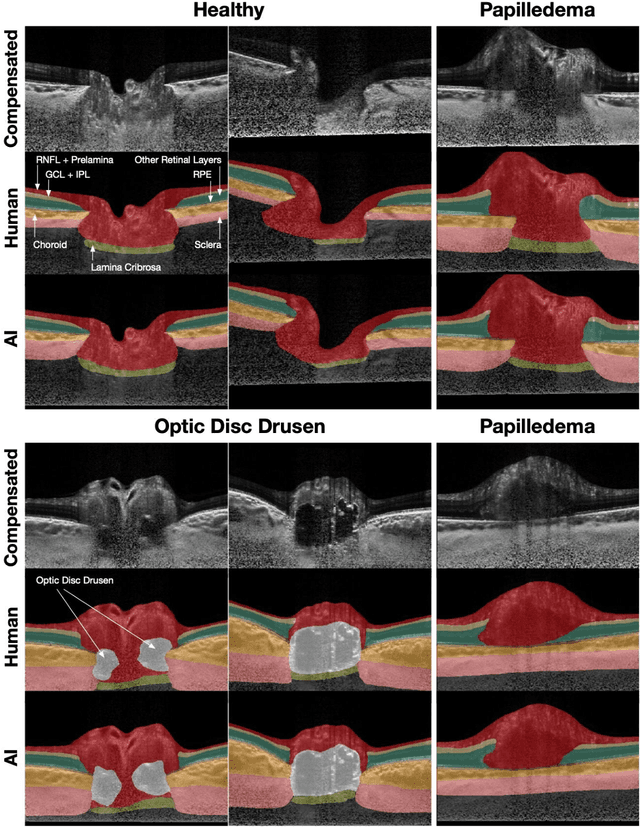
Abstract:Purpose: (1) To develop a deep learning algorithm to identify major tissue structures of the optic nerve head (ONH) in 3D optical coherence tomography (OCT) scans; (2) to exploit such information to robustly differentiate among healthy, optic disc drusen (ODD), and papilledema ONHs. It was a cross-sectional comparative study with confirmed ODD (105 eyes), papilledema due to high intracranial pressure (51 eyes), and healthy controls (100 eyes). 3D scans of the ONHs were acquired using OCT, then processed to improve deep-tissue visibility. At first, a deep learning algorithm was developed using 984 B-scans (from 130 eyes) in order to identify: major neural/connective tissues, and ODD regions. The performance of our algorithm was assessed using the Dice coefficient (DC). In a 2nd step, a classification algorithm (random forest) was designed using 150 OCT volumes to perform 3-class classifications (1: ODD, 2: papilledema, 3: healthy) strictly from their drusen and prelamina swelling scores (derived from the segmentations). To assess performance, we reported the area under the receiver operating characteristic curves (AUCs) for each class. Our segmentation algorithm was able to isolate neural and connective tissues, and ODD regions whenever present. This was confirmed by an average DC of 0.93$\pm$0.03 on the test set, corresponding to good performance. Classification was achieved with high AUCs, i.e. 0.99$\pm$0.01 for the detection of ODD, 0.99 $\pm$ 0.01 for the detection of papilledema, and 0.98$\pm$0.02 for the detection of healthy ONHs. Our AI approach accurately discriminated ODD from papilledema, using a single OCT scan. Our classification performance was excellent, with the caveat that validation in a much larger population is warranted. Our approach may have the potential to establish OCT as the mainstay of diagnostic imaging in neuro-ophthalmology.
The Three-Dimensional Structural Configuration of the Central Retinal Vessel Trunk and Branches as a Glaucoma Biomarker
Nov 09, 2021
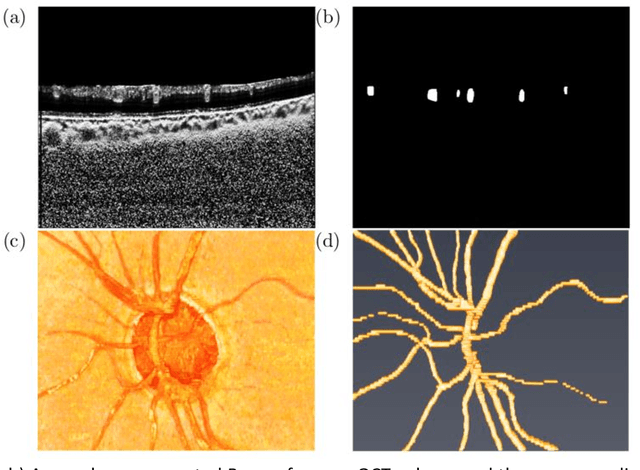
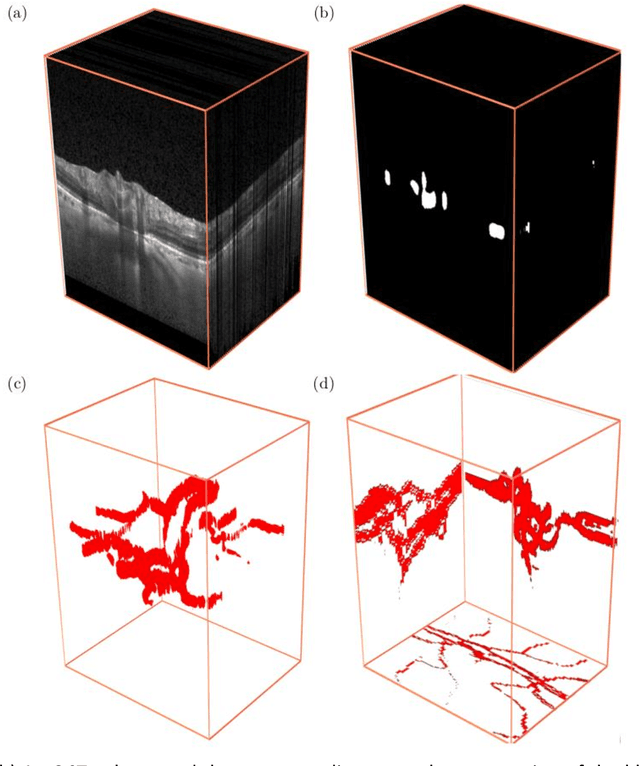

Abstract:Purpose: To assess whether the three-dimensional (3D) structural configuration of the central retinal vessel trunk and its branches (CRVT&B) could be used as a diagnostic marker for glaucoma. Method: We trained a deep learning network to automatically segment the CRVT&B from the B-scans of the optical coherence tomography (OCT) volume of the optic nerve head (ONH). Subsequently, two different approaches were used for glaucoma diagnosis using the structural configuration of the CRVT&B as extracted from the OCT volumes. In the first approach, we aimed to provide a diagnosis using only 3D CNN and the 3D structure of the CRVT&B. For the second approach, we projected the 3D structure of the CRVT&B orthographically onto three planes to obtain 2D images, and then a 2D CNN was used for diagnosis. The segmentation accuracy was evaluated using the Dice coefficient, whereas the diagnostic accuracy was assessed using the area under the receiver operating characteristic curves (AUC). The diagnostic performance of the CRVT&B was also compared with that of retinal nerve fiber layer (RNFL) thickness. Results: Our segmentation network was able to efficiently segment retinal blood vessels from OCT scans. On a test set, we achieved a Dice coefficient of 0.81\pm0.07. The 3D and 2D diagnostic networks were able to differentiate glaucoma from non-glaucoma subjects with accuracies of 82.7% and 83.3%, respectively. The corresponding AUCs for CRVT&B were 0.89 and 0.90, higher than those obtained with RNFL thickness alone. Conclusions: Our work demonstrated that the diagnostic power of the CRVT&B is superior to that of a gold-standard glaucoma parameter, i.e., RNFL thickness. Our work also suggested that the major retinal blood vessels form a skeleton -- the configuration of which may be representative of major ONH structural changes as typically observed with the development and progression of glaucoma.
Describing the Structural Phenotype of the Glaucomatous Optic Nerve Head Using Artificial Intelligence
Dec 17, 2020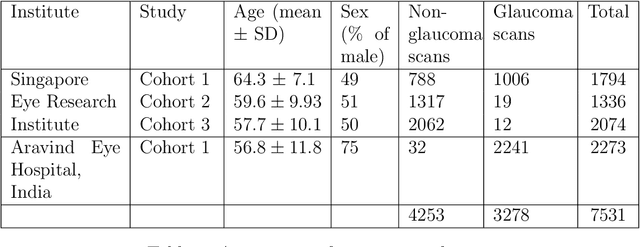
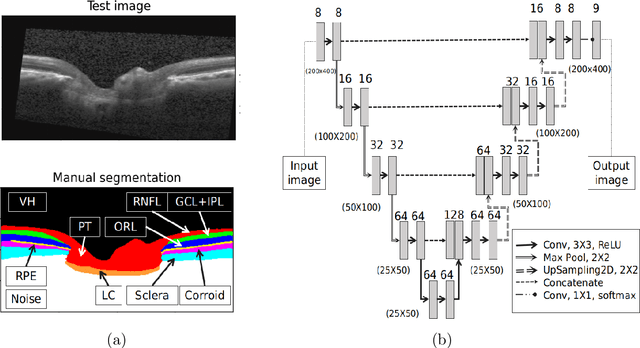
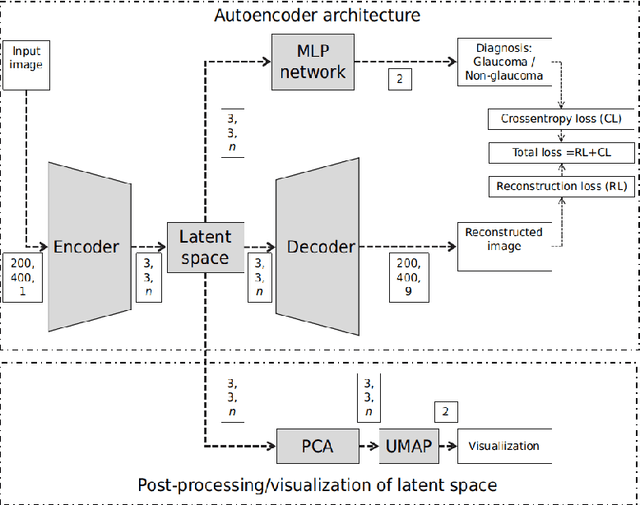

Abstract:The optic nerve head (ONH) typically experiences complex neural- and connective-tissue structural changes with the development and progression of glaucoma, and monitoring these changes could be critical for improved diagnosis and prognosis in the glaucoma clinic. The gold-standard technique to assess structural changes of the ONH clinically is optical coherence tomography (OCT). However, OCT is limited to the measurement of a few hand-engineered parameters, such as the thickness of the retinal nerve fiber layer (RNFL), and has not yet been qualified as a stand-alone device for glaucoma diagnosis and prognosis applications. We argue this is because the vast amount of information available in a 3D OCT scan of the ONH has not been fully exploited. In this study we propose a deep learning approach that can: \textbf{(1)} fully exploit information from an OCT scan of the ONH; \textbf{(2)} describe the structural phenotype of the glaucomatous ONH; and that can \textbf{(3)} be used as a robust glaucoma diagnosis tool. Specifically, the structural features identified by our algorithm were found to be related to clinical observations of glaucoma. The diagnostic accuracy from these structural features was $92.0 \pm 2.3 \%$ with a sensitivity of $90.0 \pm 2.4 \% $ (at $95 \%$ specificity). By changing their magnitudes in steps, we were able to reveal how the morphology of the ONH changes as one transitions from a `non-glaucoma' to a `glaucoma' condition. We believe our work may have strong clinical implication for our understanding of glaucoma pathogenesis, and could be improved in the future to also predict future loss of vision.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge