Martin L. Buist
3D Structural Phenotype of the Optic Nerve Head at the Intersection of Glaucoma and Myopia - A Key to Improving Glaucoma Diagnosis in Myopic Populations
Mar 24, 2025Abstract:Purpose: To characterize the 3D structural phenotypes of the optic nerve head (ONH) in patients with glaucoma, high myopia, and concurrent high myopia and glaucoma, and to evaluate their variations across these conditions. Participants: A total of 685 optical coherence tomography (OCT) scans from 754 subjects of Singapore-Chinese ethnicity, including 256 healthy (H), 94 highly myopic (HM), 227 glaucomatous (G), and 108 highly myopic with glaucoma (HMG) cases. Methods: We segmented the retinal and connective tissues from OCT volumes and their boundary edges were converted into 3D point clouds. To classify the 3D point clouds into four ONH conditions, i.e., H, HM, G, and HMG, a specialized ensemble network was developed, consisting of an encoder to transform high-dimensional input data into a compressed latent vector, a decoder to reconstruct point clouds from the latent vector, and a classifier to categorize the point clouds into the four ONH conditions. Results: The classification network achieved high accuracy, distinguishing H, HM, G, and HMG classes with a micro-average AUC of 0.92 $\pm$ 0.03 on an independent test set. The decoder effectively reconstructed point clouds, achieving a Chamfer loss of 0.013 $\pm$ 0.002. Dimensionality reduction clustered ONHs into four distinct groups, revealing structural variations such as changes in retinal and connective tissue thickness, tilting and stretching of the disc and scleral canal opening, and alterations in optic cup morphology, including shallow or deep excavation, across the four conditions. Conclusions: This study demonstrated that ONHs exhibit distinct structural signatures across H, HM, G, and HMG conditions. The findings further indicate that ONH morphology provides sufficient information for classification into distinct clusters, with principal components capturing unique structural patterns within each group.
The Three-Dimensional Structural Configuration of the Central Retinal Vessel Trunk and Branches as a Glaucoma Biomarker
Nov 09, 2021
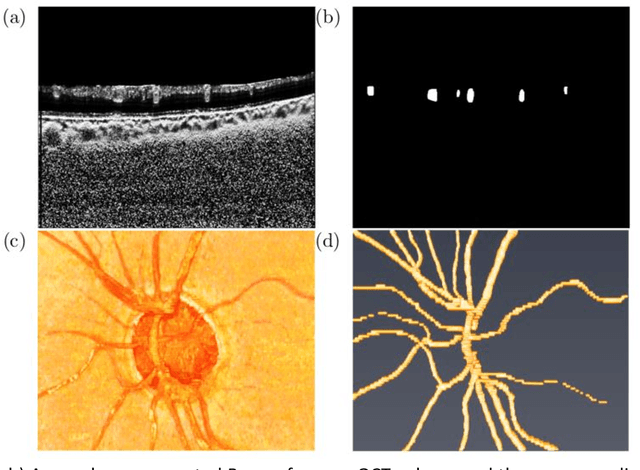
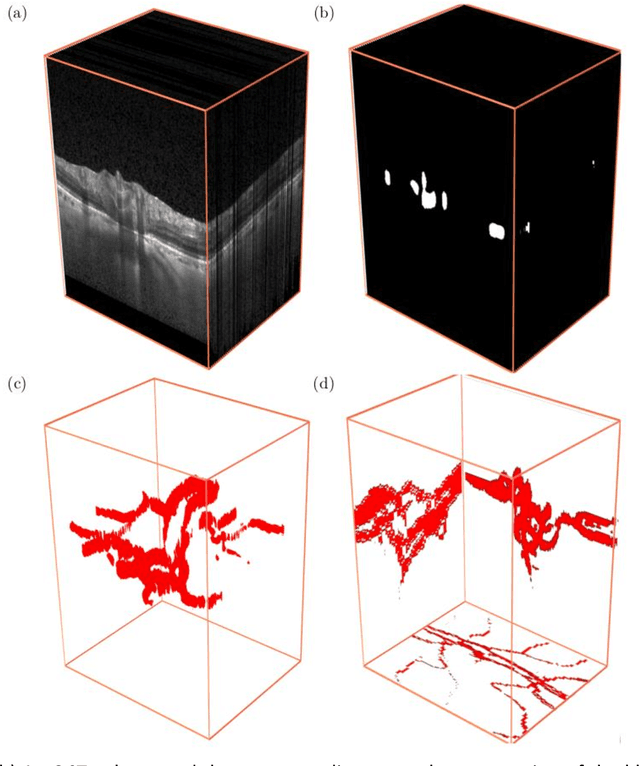

Abstract:Purpose: To assess whether the three-dimensional (3D) structural configuration of the central retinal vessel trunk and its branches (CRVT&B) could be used as a diagnostic marker for glaucoma. Method: We trained a deep learning network to automatically segment the CRVT&B from the B-scans of the optical coherence tomography (OCT) volume of the optic nerve head (ONH). Subsequently, two different approaches were used for glaucoma diagnosis using the structural configuration of the CRVT&B as extracted from the OCT volumes. In the first approach, we aimed to provide a diagnosis using only 3D CNN and the 3D structure of the CRVT&B. For the second approach, we projected the 3D structure of the CRVT&B orthographically onto three planes to obtain 2D images, and then a 2D CNN was used for diagnosis. The segmentation accuracy was evaluated using the Dice coefficient, whereas the diagnostic accuracy was assessed using the area under the receiver operating characteristic curves (AUC). The diagnostic performance of the CRVT&B was also compared with that of retinal nerve fiber layer (RNFL) thickness. Results: Our segmentation network was able to efficiently segment retinal blood vessels from OCT scans. On a test set, we achieved a Dice coefficient of 0.81\pm0.07. The 3D and 2D diagnostic networks were able to differentiate glaucoma from non-glaucoma subjects with accuracies of 82.7% and 83.3%, respectively. The corresponding AUCs for CRVT&B were 0.89 and 0.90, higher than those obtained with RNFL thickness alone. Conclusions: Our work demonstrated that the diagnostic power of the CRVT&B is superior to that of a gold-standard glaucoma parameter, i.e., RNFL thickness. Our work also suggested that the major retinal blood vessels form a skeleton -- the configuration of which may be representative of major ONH structural changes as typically observed with the development and progression of glaucoma.
Describing the Structural Phenotype of the Glaucomatous Optic Nerve Head Using Artificial Intelligence
Dec 17, 2020
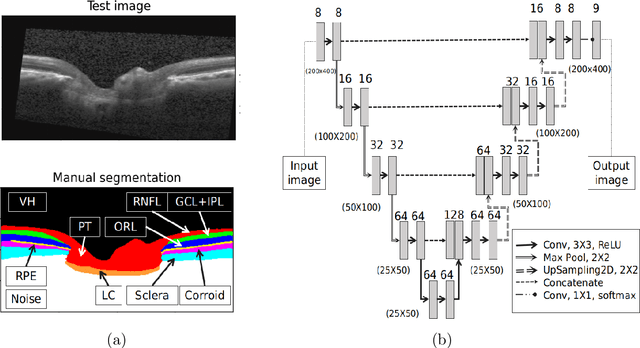
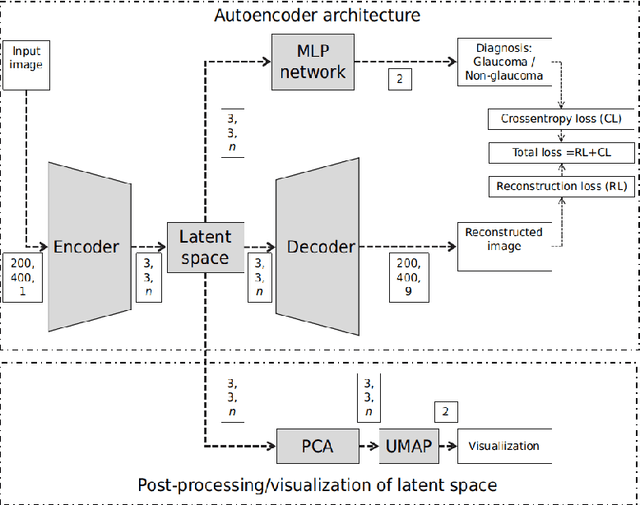

Abstract:The optic nerve head (ONH) typically experiences complex neural- and connective-tissue structural changes with the development and progression of glaucoma, and monitoring these changes could be critical for improved diagnosis and prognosis in the glaucoma clinic. The gold-standard technique to assess structural changes of the ONH clinically is optical coherence tomography (OCT). However, OCT is limited to the measurement of a few hand-engineered parameters, such as the thickness of the retinal nerve fiber layer (RNFL), and has not yet been qualified as a stand-alone device for glaucoma diagnosis and prognosis applications. We argue this is because the vast amount of information available in a 3D OCT scan of the ONH has not been fully exploited. In this study we propose a deep learning approach that can: \textbf{(1)} fully exploit information from an OCT scan of the ONH; \textbf{(2)} describe the structural phenotype of the glaucomatous ONH; and that can \textbf{(3)} be used as a robust glaucoma diagnosis tool. Specifically, the structural features identified by our algorithm were found to be related to clinical observations of glaucoma. The diagnostic accuracy from these structural features was $92.0 \pm 2.3 \%$ with a sensitivity of $90.0 \pm 2.4 \% $ (at $95 \%$ specificity). By changing their magnitudes in steps, we were able to reveal how the morphology of the ONH changes as one transitions from a `non-glaucoma' to a `glaucoma' condition. We believe our work may have strong clinical implication for our understanding of glaucoma pathogenesis, and could be improved in the future to also predict future loss of vision.
OCT-GAN: Single Step Shadow and Noise Removal from Optical Coherence Tomography Images of the Human Optic Nerve Head
Oct 06, 2020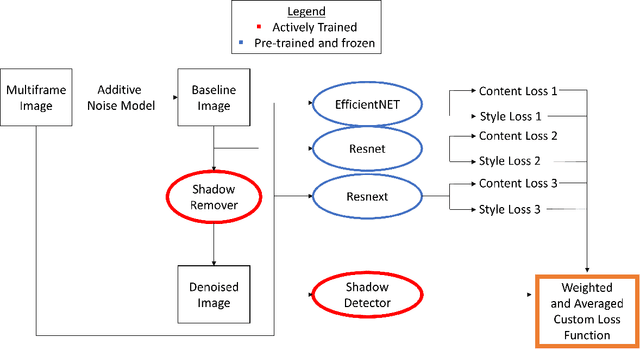
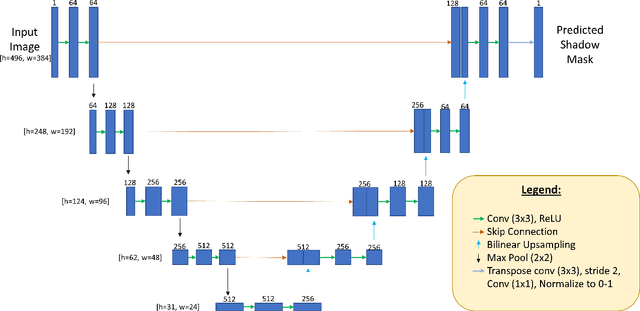
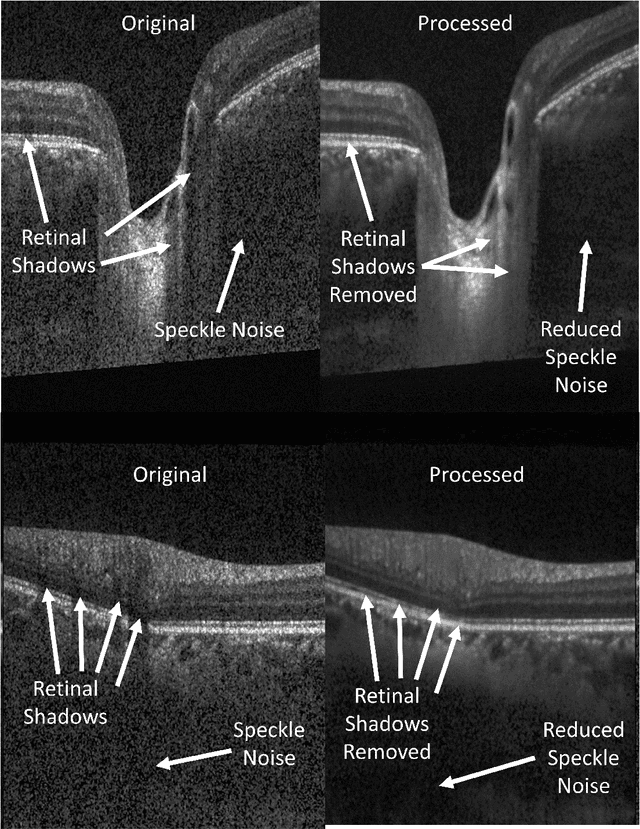

Abstract:Speckle noise and retinal shadows within OCT B-scans occlude important edges, fine textures and deep tissues, preventing accurate and robust diagnosis by algorithms and clinicians. We developed a single process that successfully removed both noise and retinal shadows from unseen single-frame B-scans within 10.4ms. Mean average gradient magnitude (AGM) for the proposed algorithm was 57.2% higher than current state-of-the-art, while mean peak signal to noise ratio (PSNR), contrast to noise ratio (CNR), and structural similarity index metric (SSIM) increased by 11.1%, 154% and 187% respectively compared to single-frame B-scans. Mean intralayer contrast (ILC) improvement for the retinal nerve fiber layer (RNFL), photoreceptor layer (PR) and retinal pigment epithelium (RPE) layers decreased from 0.362 \pm 0.133 to 0.142 \pm 0.102, 0.449 \pm 0.116 to 0.0904 \pm 0.0769, 0.381 \pm 0.100 to 0.0590 \pm 0.0451 respectively. The proposed algorithm reduces the necessity for long image acquisition times, minimizes expensive hardware requirements and reduces motion artifacts in OCT images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge