Savvas Nicolaou
A Non-contrast Head CT Foundation Model for Comprehensive Neuro-Trauma Triage
Feb 28, 2025



Abstract:Recent advancements in AI and medical imaging offer transformative potential in emergency head CT interpretation for reducing assessment times and improving accuracy in the face of an increasing request of such scans and a global shortage in radiologists. This study introduces a 3D foundation model for detecting diverse neuro-trauma findings with high accuracy and efficiency. Using large language models (LLMs) for automatic labeling, we generated comprehensive multi-label annotations for critical conditions. Our approach involved pretraining neural networks for hemorrhage subtype segmentation and brain anatomy parcellation, which were integrated into a pretrained comprehensive neuro-trauma detection network through multimodal fine-tuning. Performance evaluation against expert annotations and comparison with CT-CLIP demonstrated strong triage accuracy across major neuro-trauma findings, such as hemorrhage and midline shift, as well as less frequent critical conditions such as cerebral edema and arterial hyperdensity. The integration of neuro-specific features significantly enhanced diagnostic capabilities, achieving an average AUC of 0.861 for 16 neuro-trauma conditions. This work advances foundation models in medical imaging, serving as a benchmark for future AI-assisted neuro-trauma diagnostics in emergency radiology.
The RSNA Abdominal Traumatic Injury CT (RATIC) Dataset
May 30, 2024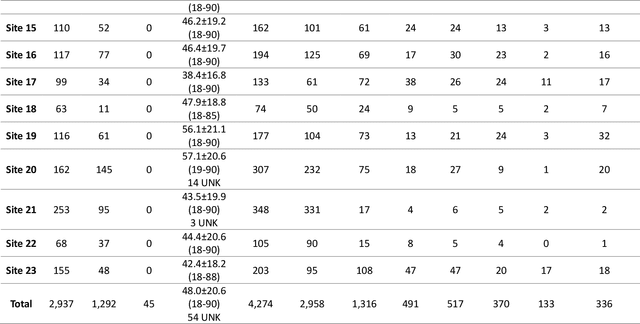
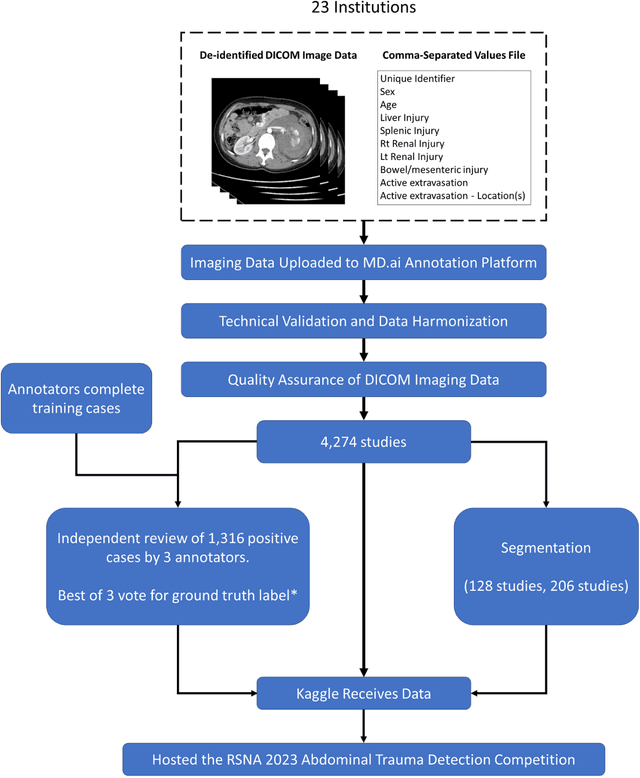
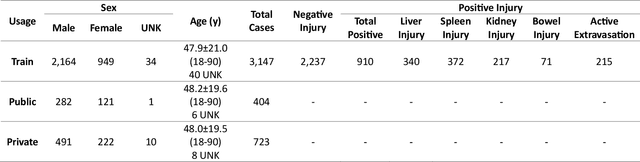
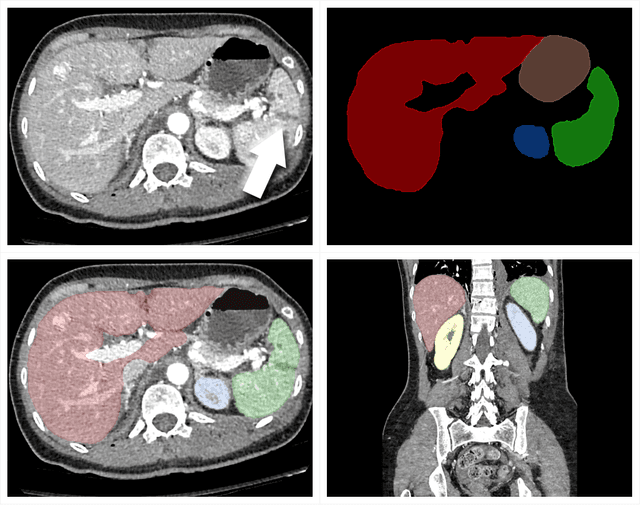
Abstract:The RSNA Abdominal Traumatic Injury CT (RATIC) dataset is the largest publicly available collection of adult abdominal CT studies annotated for traumatic injuries. This dataset includes 4,274 studies from 23 institutions across 14 countries. The dataset is freely available for non-commercial use via Kaggle at https://www.kaggle.com/competitions/rsna-2023-abdominal-trauma-detection. Created for the RSNA 2023 Abdominal Trauma Detection competition, the dataset encourages the development of advanced machine learning models for detecting abdominal injuries on CT scans. The dataset encompasses detection and classification of traumatic injuries across multiple organs, including the liver, spleen, kidneys, bowel, and mesentery. Annotations were created by expert radiologists from the American Society of Emergency Radiology (ASER) and Society of Abdominal Radiology (SAR). The dataset is annotated at multiple levels, including the presence of injuries in three solid organs with injury grading, image-level annotations for active extravasations and bowel injury, and voxelwise segmentations of each of the potentially injured organs. With the release of this dataset, we hope to facilitate research and development in machine learning and abdominal trauma that can lead to improved patient care and outcomes.
Segmentation of Pulmonary Opacification in Chest CT Scans of COVID-19 Patients
Jul 08, 2020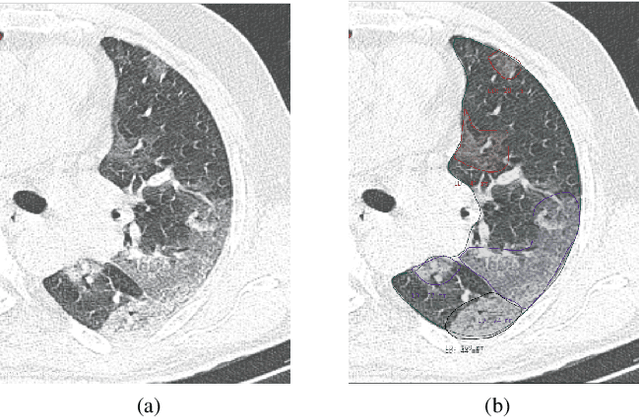
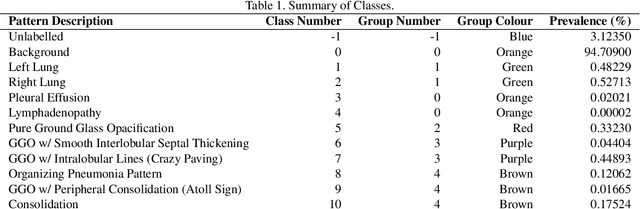
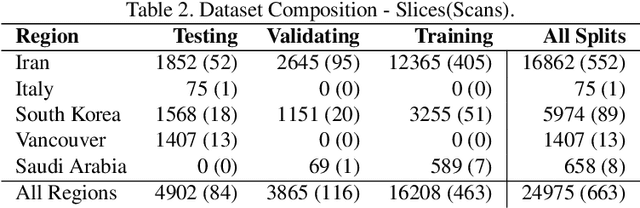
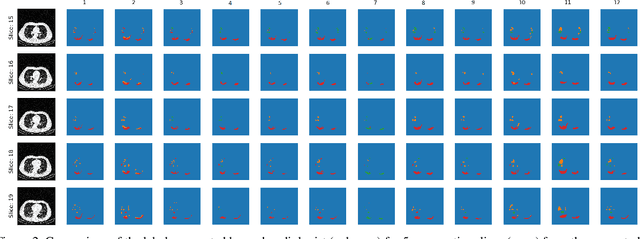
Abstract:The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has rapidly spread into a global pandemic. A form of pneumonia, presenting as opacities with in a patient's lungs, is the most common presentation associated with this virus, and great attention has gone into how these changes relate to patient morbidity and mortality. In this work we provide open source models for the segmentation of patterns of pulmonary opacification on chest Computed Tomography (CT) scans which have been correlated with various stages and severities of infection. We have collected 663 chest CT scans of COVID-19 patients from healthcare centers around the world, and created pixel wise segmentation labels for nearly 25,000 slices that segment 6 different patterns of pulmonary opacification. We provide open source implementations and pre-trained weights for multiple segmentation models trained on our dataset. Our best model achieves an opacity Intersection-Over-Union score of 0.76 on our test set, demonstrates successful domain adaptation, and predicts the volume of opacification within 1.7\% of expert radiologists. Additionally, we present an analysis of the inter-observer variability inherent to this task, and propose methods for appropriate probabilistic approaches.
Machine Learning Automatically Detects COVID-19 using Chest CTs in a Large Multicenter Cohort
Jun 11, 2020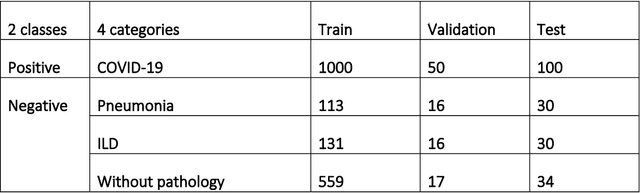
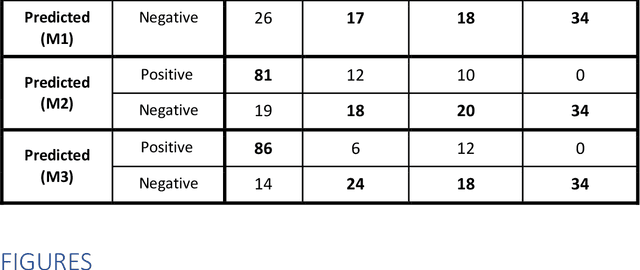
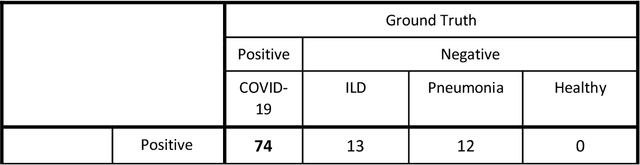
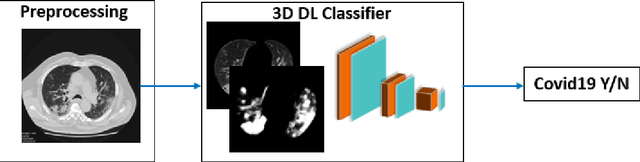
Abstract:Purpose: To investigate if AI-based classifiers can distinguish COVID-19 from other pulmonary diseases and normal groups, using chest CT images. To study the interpretability of discriminative features for COVID19 detection. Materials and Methods: Our database consists of 2096 CT exams that include CTs from 1150 COVID-19 patients. Training was performed on 1000 COVID-19, 131 ILD, 113 other pneumonias, 559 normal CTs, and testing on 100 COVID-19, 30 ILD, 30 other pneumonias, and 34 normal CTs. A metric-based approach for classification of COVID-19 used interpretable features, relying on logistic regression and random forests. A deep learning-based classifier differentiated COVID-19 based on 3D features extracted directly from CT intensities and from the probability distribution of airspace opacities. Results: Most discriminative features of COVID-19 are percentage of airspace opacity, ground glass opacities, consolidations, and peripheral and basal opacities, which coincide with the typical characterization of COVID-19 in the literature. Unsupervised hierarchical clustering compares the distribution of these features across COVID-19 and control cohorts. The metrics-based classifier achieved AUC, sensitivity, and specificity of respectively 0.85, 0.81, and 0.77. The DL-based classifier achieved AUC, sensitivity, and specificity of respectively 0.90, 0.86, and 0.81. Most of ambiguity comes from non-COVID-19 pneumonia with manifestations that overlap with COVID-19, as well as COVID-19 cases in early stages. Conclusion: A new method discriminates COVID-19 from other types of pneumonia, ILD, and normal, using quantitative patterns from chest CT. Our models balance interpretability of results and classification performance, and therefore may be useful to expedite and improve diagnosis of COVID-19.
Quantification of Tomographic Patterns associated with COVID-19 from Chest CT
Apr 28, 2020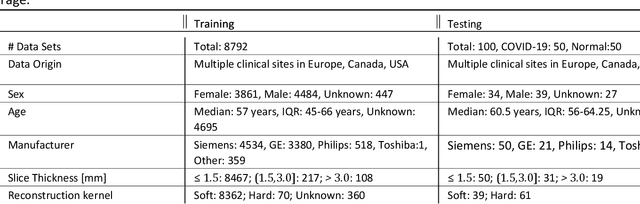
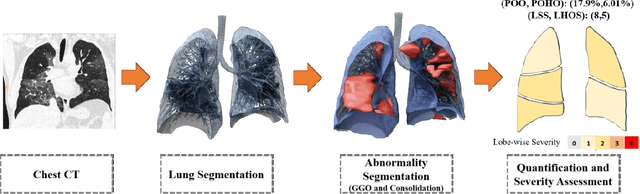
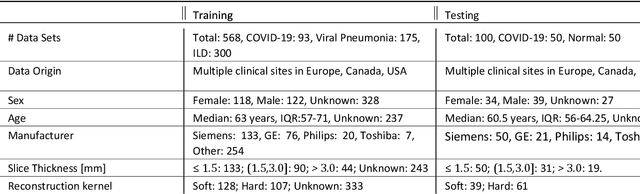
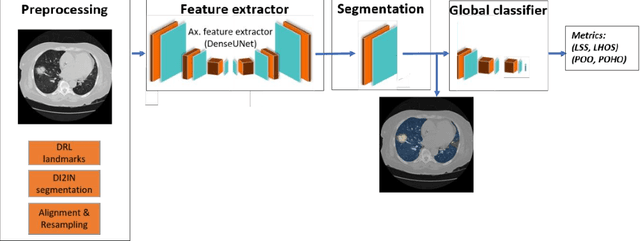
Abstract:Purpose: To present a method that automatically detects and quantifies abnormal tomographic patterns commonly present in COVID-19, namely Ground Glass Opacities (GGO) and consolidations. Given that high opacity abnormalities (i.e., consolidations) were shown to correlate with severe disease, the paper introduces two combined severity measures (Percentage of Opacity, Percentage of High Opacity) and (Lung Severity Score, Lung High Opacity Score). They quantify the extent of overall COVID-19 abnormalities and the presence of high opacity abnormalities, global and lobe-wise, respectively, being computed based on 3D segmentations of lesions, lungs, and lobes. Materials and Methods: The proposed method takes as input a non-contrasted Chest CT and segments the lesions, lungs, and lobes in 3D. It outputs two combined measures of the severity of lung/lobe involvement, quantifying both the extent of COVID-19 abnormalities and presence of high opacities, based on deep learning and deep reinforcement learning. The first measure (POO, POHO) is global, while the second (LSS, LHOS) is lobe-wise. Evaluation is reported on CTs of 100 subjects (50 COVID-19 confirmed and 50 controls) from institutions from Canada, Europe and US. Ground truth is established by manual annotations of lesions, lungs, and lobes. Results: Pearson Correlation Coefficient between method prediction and ground truth is 0.97 (POO), 0.98 (POHO), 0.96 (LSS), 0.96 (LHOS). Automated processing time to compute the severity scores is 10 seconds/case vs 30 mins needed for manual annotations. Conclusion: A new method identifies regions of abnormalities seen in COVID-19 non-contrasted Chest CT and computes (POO, POHO) and (LSS, LHOS) severity scores.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge