Reza Nezafat
$\textbf{MyoMapNet}$: Accelerated Modified Look-Locker Inversion Recovery Myocardial T1 Mapping via Neural Networks
Mar 31, 2021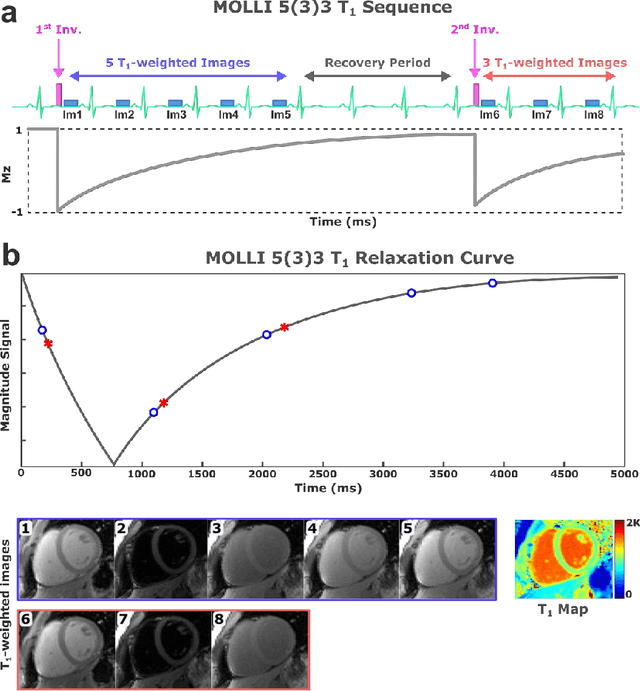
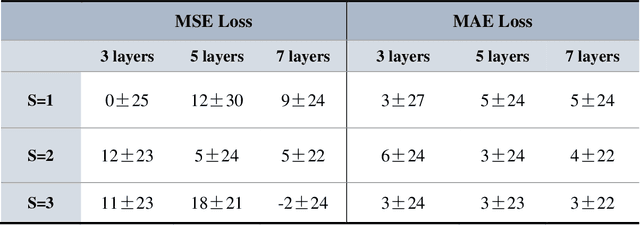
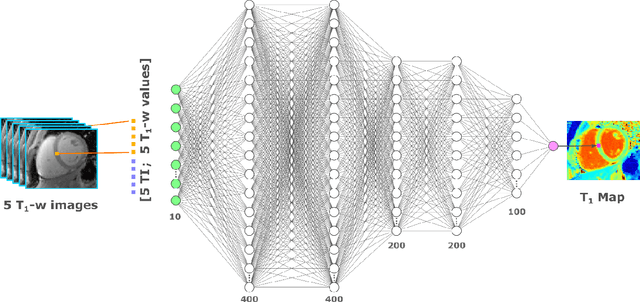
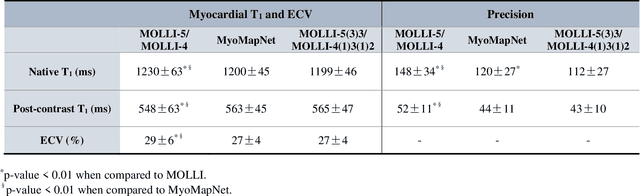
Abstract:Purpose: To develop and evaluate MyoMapNet, a rapid myocardial T1 mapping approach that uses neural networks (NN) to estimate voxel-wise myocardial T1 and extracellular (ECV) from T1-weighted images collected after a single inversion pulse over 4-5 heartbeats. Method: MyoMapNet utilizes a simple fully-connected NN to estimate T1 values from 5 (native) or 4 (post-contrast) T1-weighted images. Native MOLLI-5(3)3 T1 was collected in 717 subjects (386 males, 55$\pm$16.5 years) and post-contrast MOLLI-4(1)3(1)2 in 535 subjects (232 male, 56.5$\pm$15 years). The dataset was divided into training (80%) and testing (20%), where 20% of the training set was used to optimize MyoMapNet architecture (size and loss functions). We used MyoMapNet to estimate T1 and ECV maps with the first 5 (native) or 4 (post-contrast) T1-weighted images from the corresponding MOLLI sequence compared to the conventional and an abbreviated MOLLI using similar number of T1-weighted images with 3-parameter curve-fitting. Results: In our preliminary optimizaiton step, we determined that a 5-layers NN trained using mean-absolute-error loss yields lower estimation errors and was used subsequently in independent testing study. The myocardial T1 by MyoMapNet was similar to MOLLI (1200$\pm$45ms vs. 1199$\pm$46ms; P=0.3 for native T1, and 27.3$\pm$3.5% vs. 27.1$\pm$4%; P=0.4 for ECV). MyoMapNet had significantly smaller errors in T1 estimations compared to abbreviated-MOLLI (1$\pm$17ms vs. 31$\pm$34ms, P<0.01 for in native T1, and 0.1$\pm$1.3% vs. 1.9$\pm$2.5%, P<0.01 for ECV). The duration of T1 estimation was approximately 2 ms per slice using MyoMapNet. Conclusion: MyoMapNet T1 mapping enables myocardial T1 quantification in 4-5 heartbeats with near-instantaneous map estimation time with similar accuracy and precision as MOLLI. Keywords: Myocardial T1 mapping, MOLLI, T1 reconstruction, Neural network, Deep Learning.
Self-Supervised Physics-Guided Deep Learning Reconstruction For High-Resolution 3D LGE CMR
Nov 18, 2020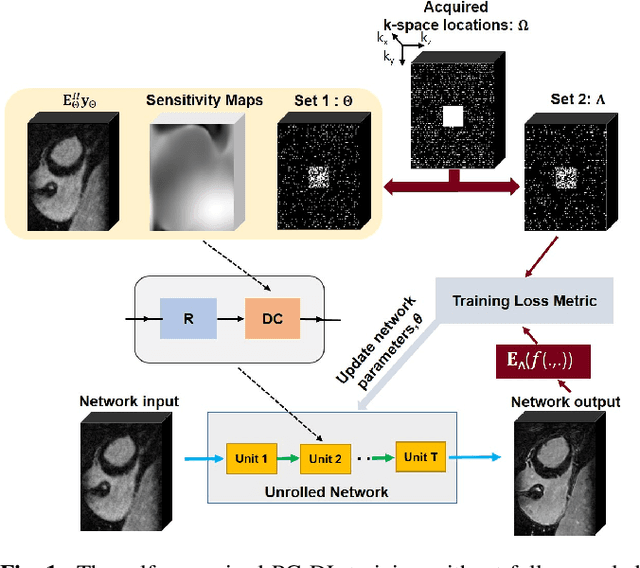
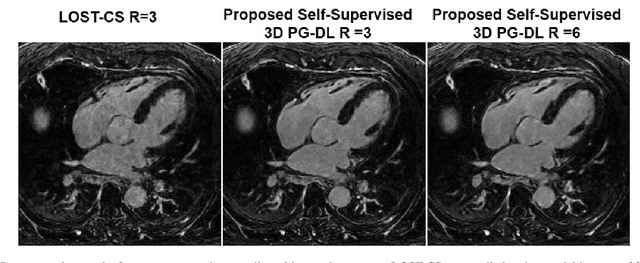
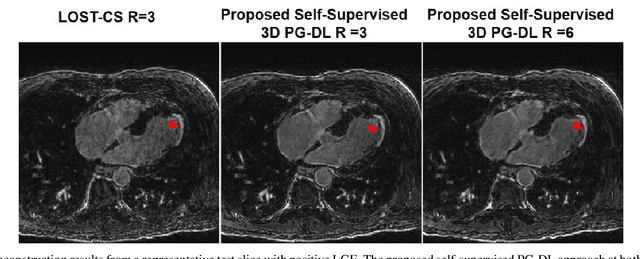
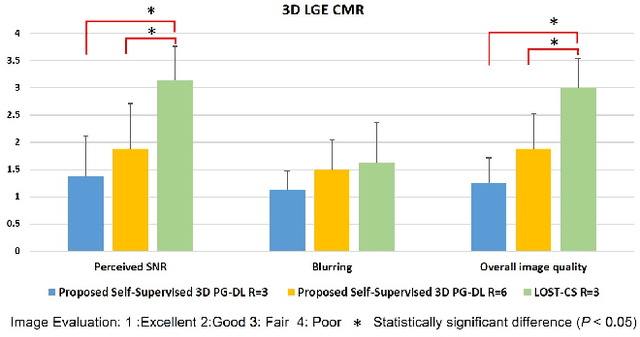
Abstract:Late gadolinium enhancement (LGE) cardiac MRI (CMR) is the clinical standard for diagnosis of myocardial scar. 3D isotropic LGE CMR provides improved coverage and resolution compared to 2D imaging. However, image acceleration is required due to long scan times and contrast washout. Physics-guided deep learning (PG-DL) approaches have recently emerged as an improved accelerated MRI strategy. Training of PG-DL methods is typically performed in supervised manner requiring fully-sampled data as reference, which is challenging in 3D LGE CMR. Recently, a self-supervised learning approach was proposed to enable training PG-DL techniques without fully-sampled data. In this work, we extend this self-supervised learning approach to 3D imaging, while tackling challenges related to small training database sizes of 3D volumes. Results and a reader study on prospectively accelerated 3D LGE show that the proposed approach at 6-fold acceleration outperforms the clinically utilized compressed sensing approach at 3-fold acceleration.
Combating Uncertainty with Novel Losses for Automatic Left Atrium Segmentation
Dec 14, 2018



Abstract:Segmenting left atrium in MR volume holds great potentials in promoting the treatment of atrial fibrillation. However, the varying anatomies, artifacts and low contrasts among tissues hinder the advance of both manual and automated solutions. In this paper, we propose a fully-automated framework to segment left atrium in gadolinium-enhanced MR volumes. The region of left atrium is firstly automatically localized by a detection module. Our framework then originates with a customized 3D deep neural network to fully explore the spatial dependency in the region for segmentation. To alleviate the risk of low training efficiency and potential overfitting, we enhance our deep network with the transfer learning and deep supervision strategy. Main contribution of our network design lies in the composite loss function to combat the boundary ambiguity and hard examples. We firstly adopt the Overlap loss to encourage network reduce the overlap between the foreground and background and thus sharpen the predictions on boundary. We then propose a novel Focal Positive loss to guide the learning of voxel-specific threshold and emphasize the foreground to improve classification sensitivity. Further improvement is obtained with an recursive training scheme. With ablation studies, all the introduced modules prove to be effective. The proposed framework achieves an average Dice of 92.24 in segmenting left atrium with pulmonary veins on 20 testing volumes.
Pyramid Network with Online Hard Example Mining for Accurate Left Atrium Segmentation
Dec 14, 2018



Abstract:Accurately segmenting left atrium in MR volume can benefit the ablation procedure of atrial fibrillation. Traditional automated solutions often fail in relieving experts from the labor-intensive manual labeling. In this paper, we propose a deep neural network based solution for automated left atrium segmentation in gadolinium-enhanced MR volumes with promising performance. We firstly argue that, for this volumetric segmentation task, networks in 2D fashion can present great superiorities in time efficiency and segmentation accuracy than networks with 3D fashion. Considering the highly varying shape of atrium and the branchy structure of associated pulmonary veins, we propose to adopt a pyramid module to collect semantic cues in feature maps from multiple scales for fine-grained segmentation. Also, to promote our network in classifying the hard examples, we propose an Online Hard Negative Example Mining strategy to identify voxels in slices with low classification certainties and penalize the wrong predictions on them. Finally, we devise a competitive training scheme to further boost the generalization ability of networks. Extensively verified on 20 testing volumes, our proposed framework achieves an average Dice of 92.83% in segmenting the left atria and pulmonary veins.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge