Qixin Hu
Analyzing Tumors by Synthesis
Sep 09, 2024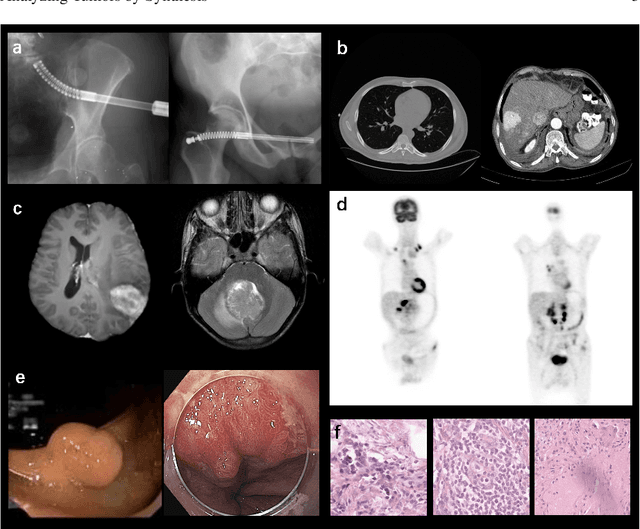
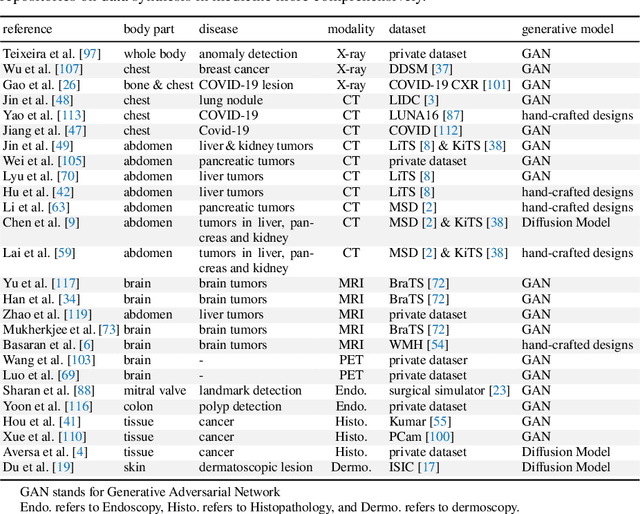
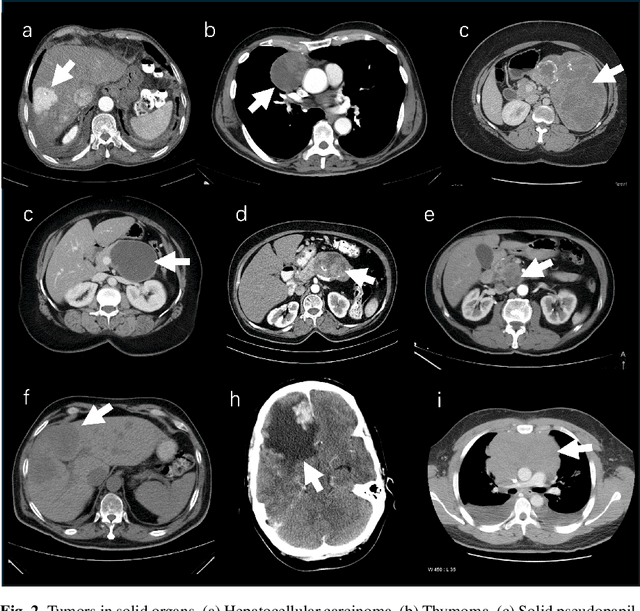
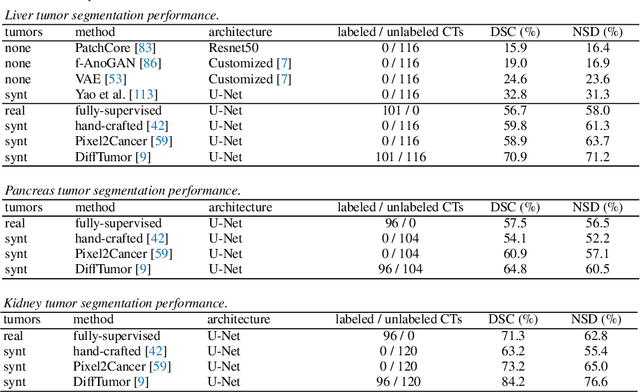
Abstract:Computer-aided tumor detection has shown great potential in enhancing the interpretation of over 80 million CT scans performed annually in the United States. However, challenges arise due to the rarity of CT scans with tumors, especially early-stage tumors. Developing AI with real tumor data faces issues of scarcity, annotation difficulty, and low prevalence. Tumor synthesis addresses these challenges by generating numerous tumor examples in medical images, aiding AI training for tumor detection and segmentation. Successful synthesis requires realistic and generalizable synthetic tumors across various organs. This chapter reviews AI development on real and synthetic data and summarizes two key trends in synthetic data for cancer imaging research: modeling-based and learning-based approaches. Modeling-based methods, like Pixel2Cancer, simulate tumor development over time using generic rules, while learning-based methods, like DiffTumor, learn from a few annotated examples in one organ to generate synthetic tumors in others. Reader studies with expert radiologists show that synthetic tumors can be convincingly realistic. We also present case studies in the liver, pancreas, and kidneys reveal that AI trained on synthetic tumors can achieve performance comparable to, or better than, AI only trained on real data. Tumor synthesis holds significant promise for expanding datasets, enhancing AI reliability, improving tumor detection performance, and preserving patient privacy.
Synthetic Data as Validation
Oct 24, 2023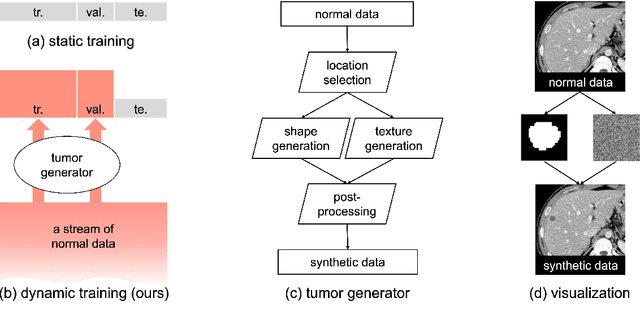
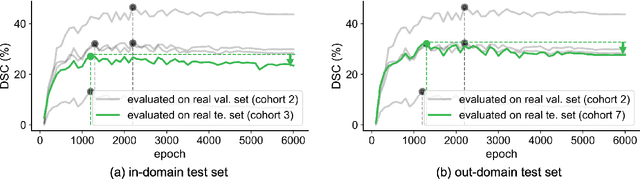
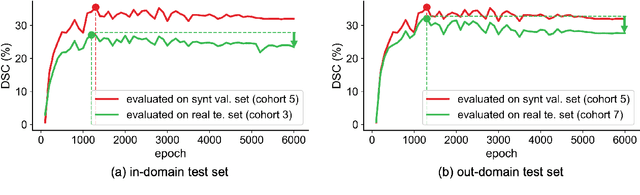
Abstract:This study leverages synthetic data as a validation set to reduce overfitting and ease the selection of the best model in AI development. While synthetic data have been used for augmenting the training set, we find that synthetic data can also significantly diversify the validation set, offering marked advantages in domains like healthcare, where data are typically limited, sensitive, and from out-domain sources (i.e., hospitals). In this study, we illustrate the effectiveness of synthetic data for early cancer detection in computed tomography (CT) volumes, where synthetic tumors are generated and superimposed onto healthy organs, thereby creating an extensive dataset for rigorous validation. Using synthetic data as validation can improve AI robustness in both in-domain and out-domain test sets. Furthermore, we establish a new continual learning framework that continuously trains AI models on a stream of out-domain data with synthetic tumors. The AI model trained and validated in dynamically expanding synthetic data can consistently outperform models trained and validated exclusively on real-world data. Specifically, the DSC score for liver tumor segmentation improves from 26.7% (95% CI: 22.6%-30.9%) to 34.5% (30.8%-38.2%) when evaluated on an in-domain dataset and from 31.1% (26.0%-36.2%) to 35.4% (32.1%-38.7%) on an out-domain dataset. Importantly, the performance gain is particularly significant in identifying very tiny liver tumors (radius < 5mm) in CT volumes, with Sensitivity improving from 33.1% to 55.4% on an in-domain dataset and 33.9% to 52.3% on an out-domain dataset, justifying the efficacy in early detection of cancer. The application of synthetic data, from both training and validation perspectives, underlines a promising avenue to enhance AI robustness when dealing with data from varying domains.
Animal3D: A Comprehensive Dataset of 3D Animal Pose and Shape
Aug 22, 2023



Abstract:Accurately estimating the 3D pose and shape is an essential step towards understanding animal behavior, and can potentially benefit many downstream applications, such as wildlife conservation. However, research in this area is held back by the lack of a comprehensive and diverse dataset with high-quality 3D pose and shape annotations. In this paper, we propose Animal3D, the first comprehensive dataset for mammal animal 3D pose and shape estimation. Animal3D consists of 3379 images collected from 40 mammal species, high-quality annotations of 26 keypoints, and importantly the pose and shape parameters of the SMAL model. All annotations were labeled and checked manually in a multi-stage process to ensure highest quality results. Based on the Animal3D dataset, we benchmark representative shape and pose estimation models at: (1) supervised learning from only the Animal3D data, (2) synthetic to real transfer from synthetically generated images, and (3) fine-tuning human pose and shape estimation models. Our experimental results demonstrate that predicting the 3D shape and pose of animals across species remains a very challenging task, despite significant advances in human pose estimation. Our results further demonstrate that synthetic pre-training is a viable strategy to boost the model performance. Overall, Animal3D opens new directions for facilitating future research in animal 3D pose and shape estimation, and is publicly available.
Label-Free Liver Tumor Segmentation
Mar 27, 2023

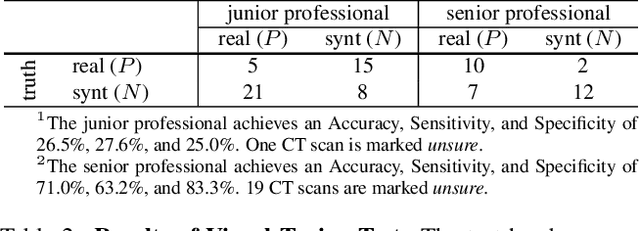
Abstract:We demonstrate that AI models can accurately segment liver tumors without the need for manual annotation by using synthetic tumors in CT scans. Our synthetic tumors have two intriguing advantages: (I) realistic in shape and texture, which even medical professionals can confuse with real tumors; (II) effective for training AI models, which can perform liver tumor segmentation similarly to the model trained on real tumors -- this result is exciting because no existing work, using synthetic tumors only, has thus far reached a similar or even close performance to real tumors. This result also implies that manual efforts for annotating tumors voxel by voxel (which took years to create) can be significantly reduced in the future. Moreover, our synthetic tumors can automatically generate many examples of small (or even tiny) synthetic tumors and have the potential to improve the success rate of detecting small liver tumors, which is critical for detecting the early stages of cancer. In addition to enriching the training data, our synthesizing strategy also enables us to rigorously assess the AI robustness.
Synthetic Tumors Make AI Segment Tumors Better
Oct 26, 2022

Abstract:We develop a novel strategy to generate synthetic tumors. Unlike existing works, the tumors generated by our strategy have two intriguing advantages: (1) realistic in shape and texture, which even medical professionals can confuse with real tumors; (2) effective for AI model training, which can perform liver tumor segmentation similarly to a model trained on real tumors - this result is unprecedented because no existing work, using synthetic tumors only, has thus far reached a similar or even close performance to the model trained on real tumors. This result also implies that manual efforts for developing per-voxel annotation of tumors (which took years to create) can be considerably reduced for training AI models in the future. Moreover, our synthetic tumors have the potential to improve the success rate of small tumor detection by automatically generating enormous examples of small (or tiny) synthetic tumors.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge