Muzaffer Özbey
Assessing the capacity of a denoising diffusion probabilistic model to reproduce spatial context
Sep 19, 2023



Abstract:Diffusion models have emerged as a popular family of deep generative models (DGMs). In the literature, it has been claimed that one class of diffusion models -- denoising diffusion probabilistic models (DDPMs) -- demonstrate superior image synthesis performance as compared to generative adversarial networks (GANs). To date, these claims have been evaluated using either ensemble-based methods designed for natural images, or conventional measures of image quality such as structural similarity. However, there remains an important need to understand the extent to which DDPMs can reliably learn medical imaging domain-relevant information, which is referred to as `spatial context' in this work. To address this, a systematic assessment of the ability of DDPMs to learn spatial context relevant to medical imaging applications is reported for the first time. A key aspect of the studies is the use of stochastic context models (SCMs) to produce training data. In this way, the ability of the DDPMs to reliably reproduce spatial context can be quantitatively assessed by use of post-hoc image analyses. Error-rates in DDPM-generated ensembles are reported, and compared to those corresponding to a modern GAN. The studies reveal new and important insights regarding the capacity of DDPMs to learn spatial context. Notably, the results demonstrate that DDPMs hold significant capacity for generating contextually correct images that are `interpolated' between training samples, which may benefit data-augmentation tasks in ways that GANs cannot.
Learning Deep MRI Reconstruction Models from Scratch in Low-Data Regimes
Jan 06, 2023Abstract:Magnetic resonance imaging (MRI) is an essential diagnostic tool that suffers from prolonged scan times. Reconstruction methods can alleviate this limitation by recovering clinically usable images from accelerated acquisitions. In particular, learning-based methods promise performance leaps by employing deep neural networks as data-driven priors. A powerful approach uses scan-specific (SS) priors that leverage information regarding the underlying physical signal model for reconstruction. SS priors are learned on each individual test scan without the need for a training dataset, albeit they suffer from computationally burdening inference with nonlinear networks. An alternative approach uses scan-general (SG) priors that instead leverage information regarding the latent features of MRI images for reconstruction. SG priors are frozen at test time for efficiency, albeit they require learning from a large training dataset. Here, we introduce a novel parallel-stream fusion model (PSFNet) that synergistically fuses SS and SG priors for performant MRI reconstruction in low-data regimes, while maintaining competitive inference times to SG methods. PSFNet implements its SG prior based on a nonlinear network, yet it forms its SS prior based on a linear network to maintain efficiency. A pervasive framework for combining multiple priors in MRI reconstruction is algorithmic unrolling that uses serially alternated projections, causing error propagation under low-data regimes. To alleviate error propagation, PSFNet combines its SS and SG priors via a novel parallel-stream architecture with learnable fusion parameters. Demonstrations are performed on multi-coil brain MRI for varying amounts of training data. PSFNet outperforms SG methods in low-data regimes, and surpasses SS methods with few tens of training samples.
Unsupervised Medical Image Translation with Adversarial Diffusion Models
Jul 17, 2022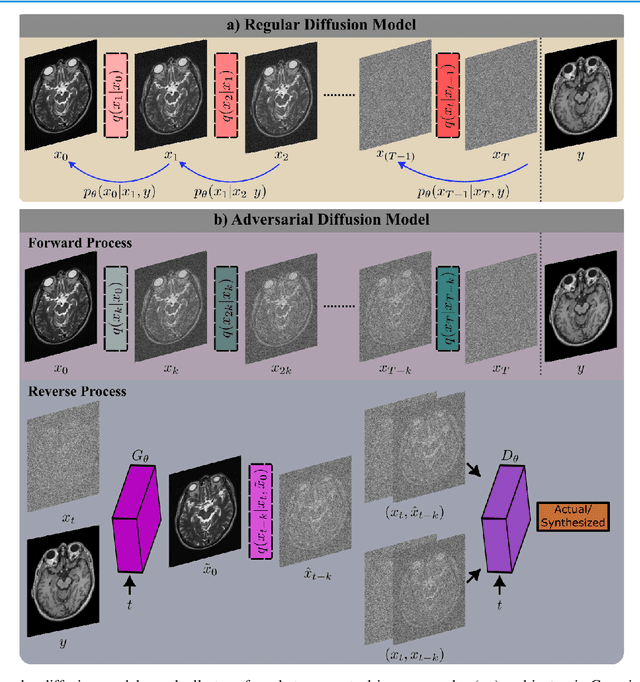
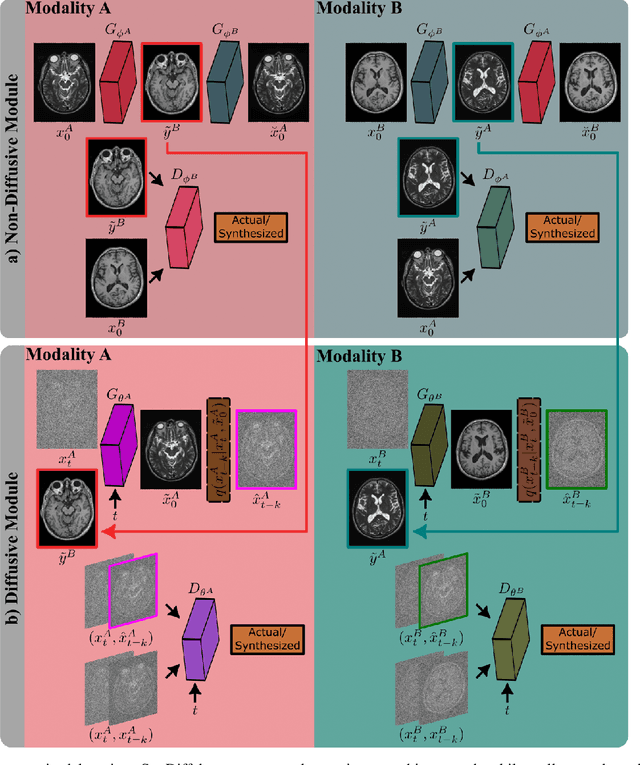
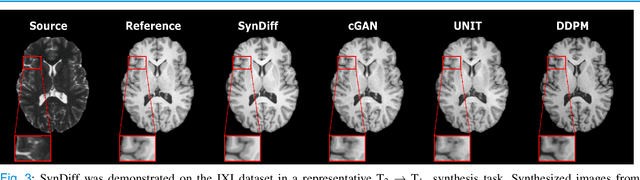
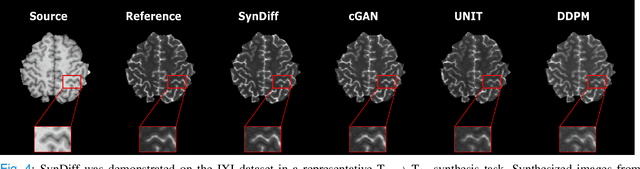
Abstract:Imputation of missing images via source-to-target modality translation can facilitate downstream tasks in medical imaging. A pervasive approach for synthesizing target images involves one-shot mapping through generative adversarial networks (GAN). Yet, GAN models that implicitly characterize the image distribution can suffer from limited sample fidelity and diversity. Here, we propose a novel method based on adversarial diffusion modeling, SynDiff, for improved reliability in medical image synthesis. To capture a direct correlate of the image distribution, SynDiff leverages a conditional diffusion process to progressively map noise and source images onto the target image. For fast and accurate image sampling during inference, large diffusion steps are coupled with adversarial projections in the reverse diffusion direction. To enable training on unpaired datasets, a cycle-consistent architecture is devised with two coupled diffusion processes to synthesize the target given source and the source given target. Extensive assessments are reported on the utility of SynDiff against competing GAN and diffusion models in multi-contrast MRI and MRI-CT translation. Our demonstrations indicate that SynDiff offers superior performance against competing baselines both qualitatively and quantitatively.
One Model to Unite Them All: Personalized Federated Learning of Multi-Contrast MRI Synthesis
Jul 13, 2022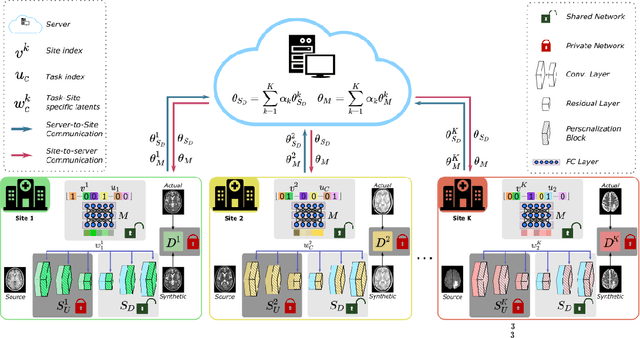
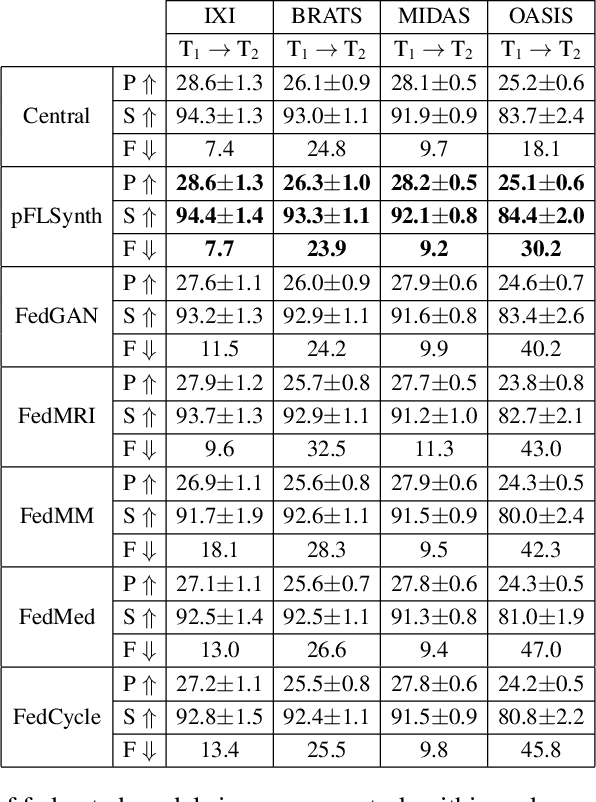
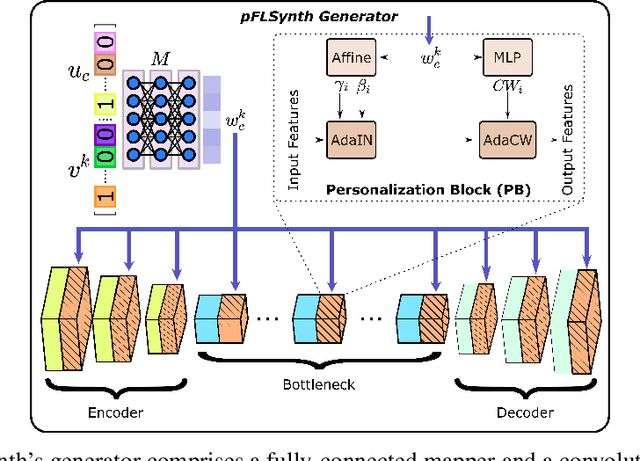
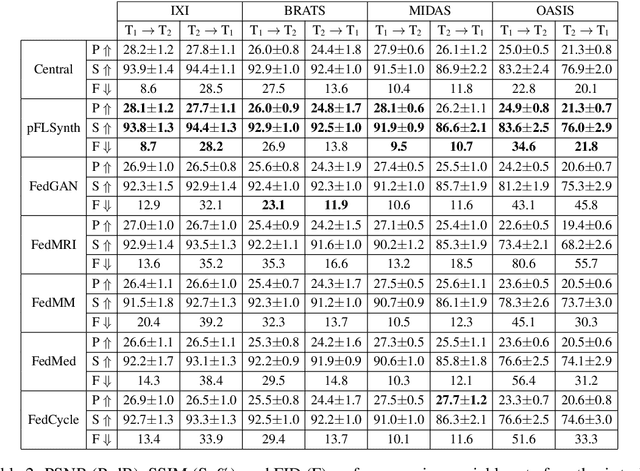
Abstract:Learning-based MRI translation involves a synthesis model that maps a source-contrast onto a target-contrast image. Multi-institutional collaborations are key to training synthesis models across broad datasets, yet centralized training involves privacy risks. Federated learning (FL) is a collaboration framework that instead adopts decentralized training to avoid sharing imaging data and mitigate privacy concerns. However, FL-trained models can be impaired by the inherent heterogeneity in the distribution of imaging data. On the one hand, implicit shifts in image distribution are evident across sites, even for a common translation task with fixed source-target configuration. Conversely, explicit shifts arise within and across sites when diverse translation tasks with varying source-target configurations are prescribed. To improve reliability against domain shifts, here we introduce the first personalized FL method for MRI Synthesis (pFLSynth). pFLSynth is based on an adversarial model equipped with a mapper that produces latents specific to individual sites and source-target contrasts. It leverages novel personalization blocks that adaptively tune the statistics and weighting of feature maps across the generator based on these latents. To further promote site-specificity, partial model aggregation is employed over downstream layers of the generator while upstream layers are retained locally. As such, pFLSynth enables training of a unified synthesis model that can reliably generalize across multiple sites and translation tasks. Comprehensive experiments on multi-site datasets clearly demonstrate the enhanced performance of pFLSynth against prior federated methods in multi-contrast MRI synthesis.
Adaptive Diffusion Priors for Accelerated MRI Reconstruction
Jul 12, 2022
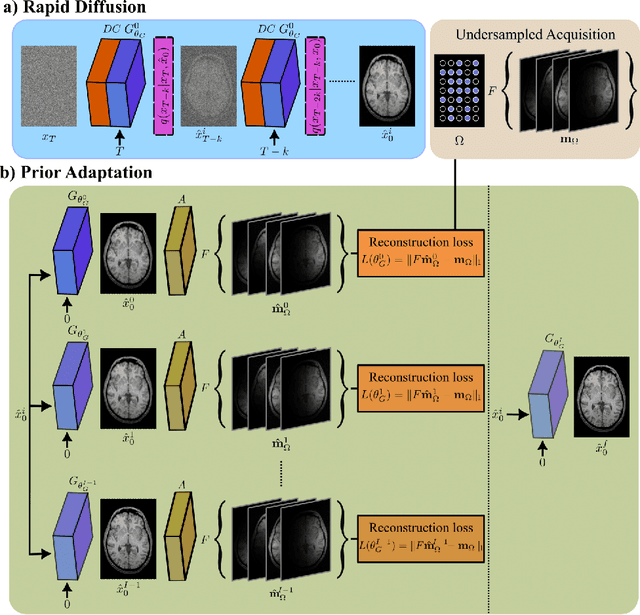
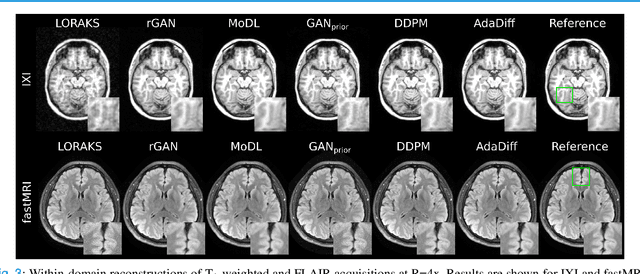
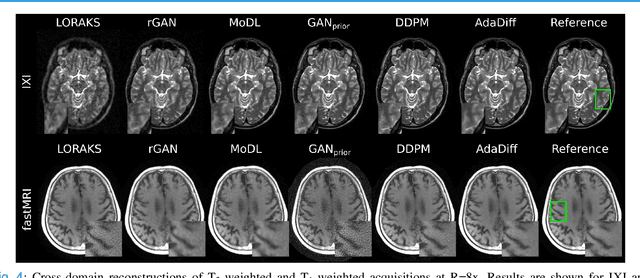
Abstract:Deep MRI reconstruction is commonly performed with conditional models that map undersampled data as input onto fully-sampled data as output. Conditional models perform de-aliasing under knowledge of the accelerated imaging operator, so they poorly generalize under domain shifts in the operator. Unconditional models are a powerful alternative that instead learn generative image priors to improve reliability against domain shifts. Recent diffusion models are particularly promising given their high representational diversity and sample quality. Nevertheless, projections through a static image prior can lead to suboptimal performance. Here we propose a novel MRI reconstruction, AdaDiff, based on an adaptive diffusion prior. To enable efficient image sampling, an adversarial mapper is introduced that enables use of large diffusion steps. A two-phase reconstruction is performed with the trained prior: a rapid-diffusion phase that produces an initial reconstruction, and an adaptation phase where the diffusion prior is updated to minimize reconstruction loss on acquired k-space data. Demonstrations on multi-contrast brain MRI clearly indicate that AdaDiff achieves superior performance to competing models in cross-domain tasks, and superior or on par performance in within-domain tasks.
Federated Learning of Generative Image Priors for MRI Reconstruction
Feb 08, 2022



Abstract:Multi-institutional efforts can facilitate training of deep MRI reconstruction models, albeit privacy risks arise during cross-site sharing of imaging data. Federated learning (FL) has recently been introduced to address privacy concerns by enabling distributed training without transfer of imaging data. Existing FL methods for MRI reconstruction employ conditional models to map from undersampled to fully-sampled acquisitions via explicit knowledge of the imaging operator. Since conditional models generalize poorly across different acceleration rates or sampling densities, imaging operators must be fixed between training and testing, and they are typically matched across sites. To improve generalization and flexibility in multi-institutional collaborations, here we introduce a novel method for MRI reconstruction based on Federated learning of Generative IMage Priors (FedGIMP). FedGIMP leverages a two-stage approach: cross-site learning of a generative MRI prior, and subject-specific injection of the imaging operator. The global MRI prior is learned via an unconditional adversarial model that synthesizes high-quality MR images based on latent variables. Specificity in the prior is preserved via a mapper subnetwork that produces site-specific latents. During inference, the prior is combined with subject-specific imaging operators to enable reconstruction, and further adapted to individual test samples by minimizing data-consistency loss. Comprehensive experiments on multi-institutional datasets clearly demonstrate enhanced generalization performance of FedGIMP against site-specific and federated methods based on conditional models, as well as traditional reconstruction methods.
Unsupervised MRI Reconstruction via Zero-Shot Learned Adversarial Transformers
May 21, 2021



Abstract:Supervised deep learning has swiftly become a workhorse for accelerated MRI in recent years, offering state-of-the-art performance in image reconstruction from undersampled acquisitions. Training deep supervised models requires large datasets of undersampled and fully-sampled acquisitions typically from a matching set of subjects. Given scarce access to large medical datasets, this limitation has sparked interest in unsupervised methods that reduce reliance on fully-sampled ground-truth data. A common framework is based on the deep image prior, where network-driven regularization is enforced directly during inference on undersampled acquisitions. Yet, canonical convolutional architectures are suboptimal in capturing long-range relationships, and randomly initialized networks may hamper convergence. To address these limitations, here we introduce a novel unsupervised MRI reconstruction method based on zero-Shot Learned Adversarial TransformERs (SLATER). SLATER embodies a deep adversarial network with cross-attention transformer blocks to map noise and latent variables onto MR images. This unconditional network learns a high-quality MRI prior in a self-supervised encoding task. A zero-shot reconstruction is performed on undersampled test data, where inference is performed by optimizing network parameters, latent and noise variables to ensure maximal consistency to multi-coil MRI data. Comprehensive experiments on brain MRI datasets clearly demonstrate the superior performance of SLATER against several state-of-the-art unsupervised methods.
Three Dimensional MR Image Synthesis with Progressive Generative Adversarial Networks
Dec 18, 2020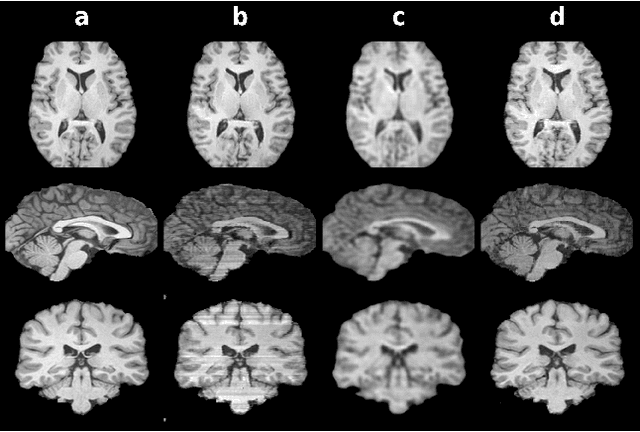
Abstract:Mainstream deep models for three-dimensional MRI synthesis are either cross-sectional or volumetric depending on the input. Cross-sectional models can decrease the model complexity, but they may lead to discontinuity artifacts. On the other hand, volumetric models can alleviate the discontinuity artifacts, but they might suffer from loss of spatial resolution due to increased model complexity coupled with scarce training data. To mitigate the limitations of both approaches, we propose a novel model that progressively recovers the target volume via simpler synthesis tasks across individual orientations.
Progressively Volumetrized Deep Generative Models for Data-Efficient Contextual Learning of MR Image Recovery
Dec 03, 2020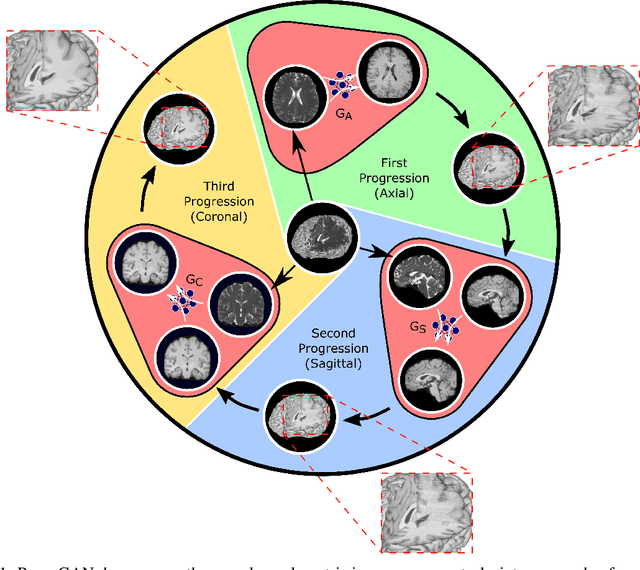
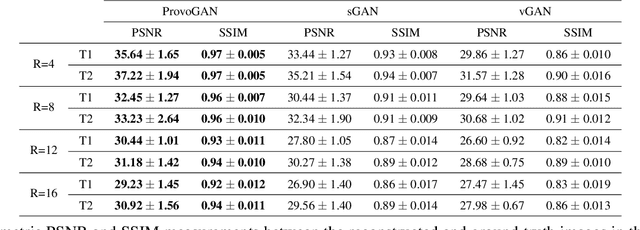
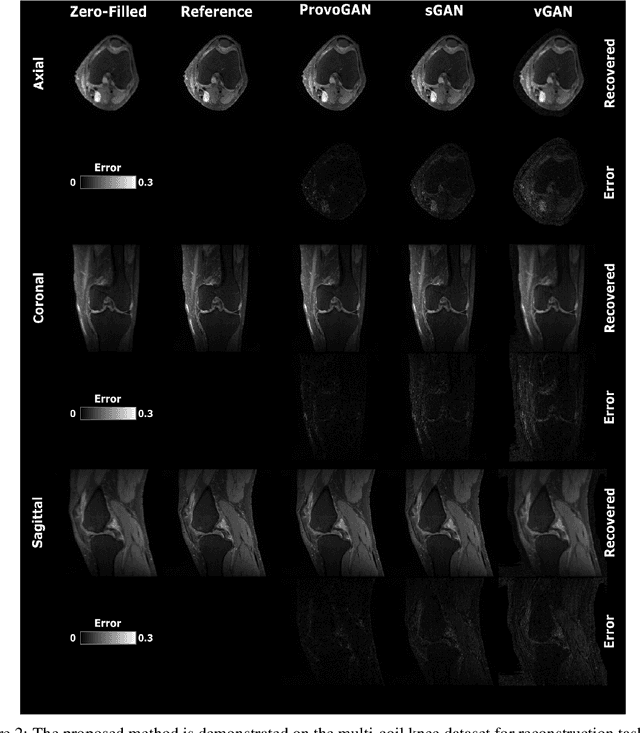
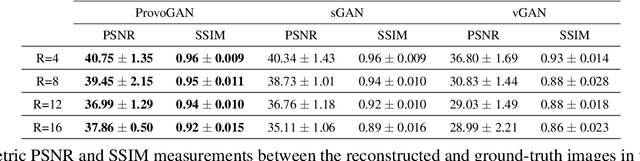
Abstract:Magnetic resonance imaging (MRI) offers the flexibility to image a given anatomic volume under a multitude of tissue contrasts. Yet, scan time considerations put stringent limits on the quality and diversity of MRI data. The gold-standard approach to alleviate this limitation is to recover high-quality images from data undersampled across various dimensions such as the Fourier domain or contrast sets. A central divide among recovery methods is whether the anatomy is processed per volume or per cross-section. Volumetric models offer enhanced capture of global contextual information, but they can suffer from suboptimal learning due to elevated model complexity. Cross-sectional models with lower complexity offer improved learning behavior, yet they ignore contextual information across the longitudinal dimension of the volume. Here, we introduce a novel data-efficient progressively volumetrized generative model (ProvoGAN) that decomposes complex volumetric image recovery tasks into a series of simpler cross-sectional tasks across individual rectilinear dimensions. ProvoGAN effectively captures global context and recovers fine-structural details across all dimensions, while maintaining low model complexity and data-efficiency advantages of cross-sectional models. Comprehensive demonstrations on mainstream MRI reconstruction and synthesis tasks show that ProvoGAN yields superior performance to state-of-the-art volumetric and cross-sectional models.
Semi-Supervised Learning of Mutually Accelerated Multi-Contrast MRI Synthesis without Fully-Sampled Ground-Truths
Nov 29, 2020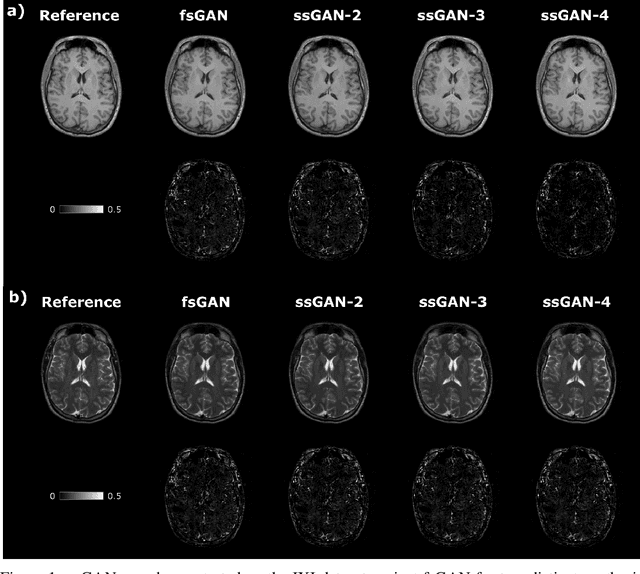
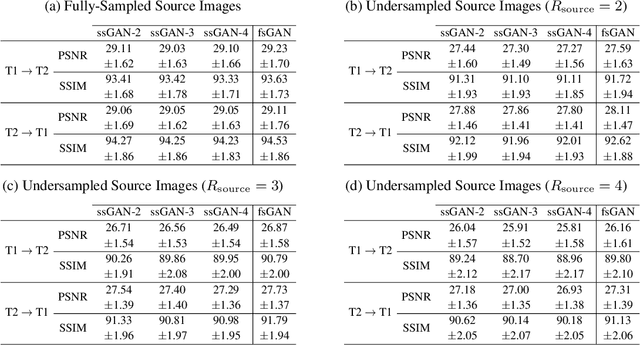
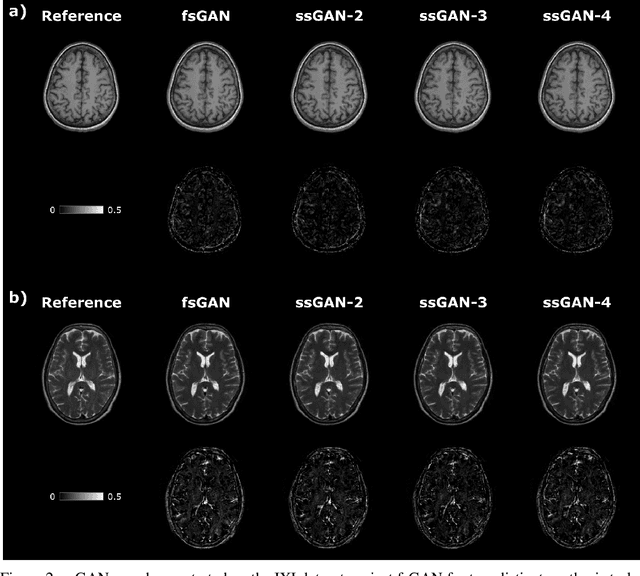
Abstract:This study proposes a novel semi-supervised learning framework for mutually accelerated multi-contrast MRI synthesis that recovers high-quality images without demanding large training sets of costly fully-sampled source or ground-truth target images. The proposed method presents a selective loss function expressed only on a subset of the acquired k-space coefficients and further leverages randomized sampling patterns across training subjects to effectively learn relationships among acquired and nonacquired k-space coefficients at all locations. Comprehensive experiments performed on multi-contrast brain images clearly demonstrate that the proposed method maintains equivalent performance to the gold-standard method based on fully-supervised training while alleviating undesirable reliance of the current synthesis methods on large-scale fully-sampled MRI acquisitions.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge