Moon Hyung Choi
Mask Enhanced Deeply Supervised Prostate Cancer Detection on B-mode Micro-Ultrasound
Dec 14, 2024



Abstract:Prostate cancer is a leading cause of cancer-related deaths among men. The recent development of high frequency, micro-ultrasound imaging offers improved resolution compared to conventional ultrasound and potentially a better ability to differentiate clinically significant cancer from normal tissue. However, the features of prostate cancer remain subtle, with ambiguous borders with normal tissue and large variations in appearance, making it challenging for both machine learning and humans to localize it on micro-ultrasound images. We propose a novel Mask Enhanced Deeply-supervised Micro-US network, termed MedMusNet, to automatically and more accurately segment prostate cancer to be used as potential targets for biopsy procedures. MedMusNet leverages predicted masks of prostate cancer to enforce the learned features layer-wisely within the network, reducing the influence of noise and improving overall consistency across frames. MedMusNet successfully detected 76% of clinically significant cancer with a Dice Similarity Coefficient of 0.365, significantly outperforming the baseline Swin-M2F in specificity and accuracy (Wilcoxon test, Bonferroni correction, p-value<0.05). While the lesion-level and patient-level analyses showed improved performance compared to human experts and different baseline, the improvements did not reach statistical significance, likely on account of the small cohort. We have presented a novel approach to automatically detect and segment clinically significant prostate cancer on B-mode micro-ultrasound images. Our MedMusNet model outperformed other models, surpassing even human experts. These preliminary results suggest the potential for aiding urologists in prostate cancer diagnosis via biopsy and treatment decision-making.
Deep Learning-based Unsupervised Domain Adaptation via a Unified Model for Prostate Lesion Detection Using Multisite Bi-parametric MRI Datasets
Aug 08, 2024


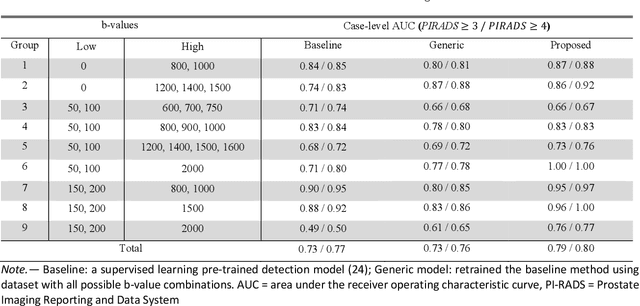
Abstract:Our hypothesis is that UDA using diffusion-weighted images, generated with a unified model, offers a promising and reliable strategy for enhancing the performance of supervised learning models in multi-site prostate lesion detection, especially when various b-values are present. This retrospective study included data from 5,150 patients (14,191 samples) collected across nine different imaging centers. A novel UDA method using a unified generative model was developed for multi-site PCa detection. This method translates diffusion-weighted imaging (DWI) acquisitions, including apparent diffusion coefficient (ADC) and individual DW images acquired using various b-values, to align with the style of images acquired using b-values recommended by Prostate Imaging Reporting and Data System (PI-RADS) guidelines. The generated ADC and DW images replace the original images for PCa detection. An independent set of 1,692 test cases (2,393 samples) was used for evaluation. The area under the receiver operating characteristic curve (AUC) was used as the primary metric, and statistical analysis was performed via bootstrapping. For all test cases, the AUC values for baseline SL and UDA methods were 0.73 and 0.79 (p<.001), respectively, for PI-RADS>=3, and 0.77 and 0.80 (p<.001) for PI-RADS>=4 PCa lesions. In the 361 test cases under the most unfavorable image acquisition setting, the AUC values for baseline SL and UDA were 0.49 and 0.76 (p<.001) for PI-RADS>=3, and 0.50 and 0.77 (p<.001) for PI-RADS>=4 PCa lesions. The results indicate the proposed UDA with generated images improved the performance of SL methods in multi-site PCa lesion detection across datasets with various b values, especially for images acquired with significant deviations from the PI-RADS recommended DWI protocol (e.g. with an extremely high b-value).
A Unified Multi-Phase CT Synthesis and Classification Framework for Kidney Cancer Diagnosis with Incomplete Data
Dec 09, 2023Abstract:Multi-phase CT is widely adopted for the diagnosis of kidney cancer due to the complementary information among phases. However, the complete set of multi-phase CT is often not available in practical clinical applications. In recent years, there have been some studies to generate the missing modality image from the available data. Nevertheless, the generated images are not guaranteed to be effective for the diagnosis task. In this paper, we propose a unified framework for kidney cancer diagnosis with incomplete multi-phase CT, which simultaneously recovers missing CT images and classifies cancer subtypes using the completed set of images. The advantage of our framework is that it encourages a synthesis model to explicitly learn to generate missing CT phases that are helpful for classifying cancer subtypes. We further incorporate lesion segmentation network into our framework to exploit lesion-level features for effective cancer classification in the whole CT volumes. The proposed framework is based on fully 3D convolutional neural networks to jointly optimize both synthesis and classification of 3D CT volumes. Extensive experiments on both in-house and external datasets demonstrate the effectiveness of our framework for the diagnosis with incomplete data compared with state-of-the-art baselines. In particular, cancer subtype classification using the completed CT data by our method achieves higher performance than the classification using the given incomplete data.
* This article has been accepted for publication in IEEE Journal of Biomedical and Health Informatics
ProsDectNet: Bridging the Gap in Prostate Cancer Detection via Transrectal B-mode Ultrasound Imaging
Dec 08, 2023



Abstract:Interpreting traditional B-mode ultrasound images can be challenging due to image artifacts (e.g., shadowing, speckle), leading to low sensitivity and limited diagnostic accuracy. While Magnetic Resonance Imaging (MRI) has been proposed as a solution, it is expensive and not widely available. Furthermore, most biopsies are guided by Transrectal Ultrasound (TRUS) alone and can miss up to 52% cancers, highlighting the need for improved targeting. To address this issue, we propose ProsDectNet, a multi-task deep learning approach that localizes prostate cancer on B-mode ultrasound. Our model is pre-trained using radiologist-labeled data and fine-tuned using biopsy-confirmed labels. ProsDectNet includes a lesion detection and patch classification head, with uncertainty minimization using entropy to improve model performance and reduce false positive predictions. We trained and validated ProsDectNet using a cohort of 289 patients who underwent MRI-TRUS fusion targeted biopsy. We then tested our approach on a group of 41 patients and found that ProsDectNet outperformed the average expert clinician in detecting prostate cancer on B-mode ultrasound images, achieving a patient-level ROC-AUC of 82%, a sensitivity of 74%, and a specificity of 67%. Our results demonstrate that ProsDectNet has the potential to be used as a computer-aided diagnosis system to improve targeted biopsy and treatment planning.
Multi-frame-based Cross-domain Image Denoising for Low-dose Computed Tomography
Apr 27, 2023Abstract:Computed tomography (CT) has been used worldwide for decades as one of the most important non-invasive tests in assisting diagnosis. However, the ionizing nature of X-ray exposure raises concerns about potential health risks such as cancer. The desire for lower radiation dose has driven researchers to improve the reconstruction quality, especially by removing noise and artifacts. Although previous studies on low-dose computed tomography (LDCT) denoising have demonstrated the potential of learning-based methods, most of them were developed on the simulated data collected using Radon transform. However, the real-world scenario significantly differs from the simulation domain, and the joint optimization of denoising with the modern CT image reconstruction pipeline is still missing. In this paper, for the commercially available third-generation multi-slice spiral CT scanners, we propose a two-stage method that better exploits the complete reconstruction pipeline for LDCT denoising across different domains. Our method makes good use of the high redundancy of both the multi-slice projections and the volumetric reconstructions while avoiding the collapse of information in conventional cascaded frameworks. The dedicated design also provides a clearer interpretation of the workflow. Through extensive evaluations, we demonstrate its superior performance against state-of-the-art methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge