Jimin Kim
$S^2M^2$: Scalable Stereo Matching Model for Reliable Depth Estimation
Jul 17, 2025Abstract:The pursuit of a generalizable stereo matching model, capable of performing across varying resolutions and disparity ranges without dataset-specific fine-tuning, has revealed a fundamental trade-off. Iterative local search methods achieve high scores on constrained benchmarks, but their core mechanism inherently limits the global consistency required for true generalization. On the other hand, global matching architectures, while theoretically more robust, have been historically rendered infeasible by prohibitive computational and memory costs. We resolve this dilemma with $S^2M^2$: a global matching architecture that achieves both state-of-the-art accuracy and high efficiency without relying on cost volume filtering or deep refinement stacks. Our design integrates a multi-resolution transformer for robust long-range correspondence, trained with a novel loss function that concentrates probability on feasible matches. This approach enables a more robust joint estimation of disparity, occlusion, and confidence. $S^2M^2$ establishes a new state of the art on the Middlebury v3 and ETH3D benchmarks, significantly outperforming prior methods across most metrics while reconstructing high-quality details with competitive efficiency.
Multi-frame-based Cross-domain Image Denoising for Low-dose Computed Tomography
Apr 27, 2023Abstract:Computed tomography (CT) has been used worldwide for decades as one of the most important non-invasive tests in assisting diagnosis. However, the ionizing nature of X-ray exposure raises concerns about potential health risks such as cancer. The desire for lower radiation dose has driven researchers to improve the reconstruction quality, especially by removing noise and artifacts. Although previous studies on low-dose computed tomography (LDCT) denoising have demonstrated the potential of learning-based methods, most of them were developed on the simulated data collected using Radon transform. However, the real-world scenario significantly differs from the simulation domain, and the joint optimization of denoising with the modern CT image reconstruction pipeline is still missing. In this paper, for the commercially available third-generation multi-slice spiral CT scanners, we propose a two-stage method that better exploits the complete reconstruction pipeline for LDCT denoising across different domains. Our method makes good use of the high redundancy of both the multi-slice projections and the volumetric reconstructions while avoiding the collapse of information in conventional cascaded frameworks. The dedicated design also provides a clearer interpretation of the workflow. Through extensive evaluations, we demonstrate its superior performance against state-of-the-art methods.
Deep Reinforcement Learning for Neural Control
Jun 12, 2020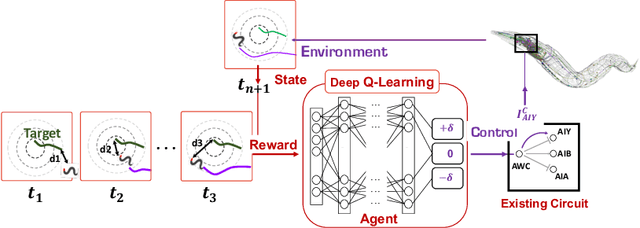
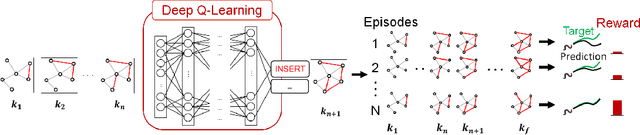
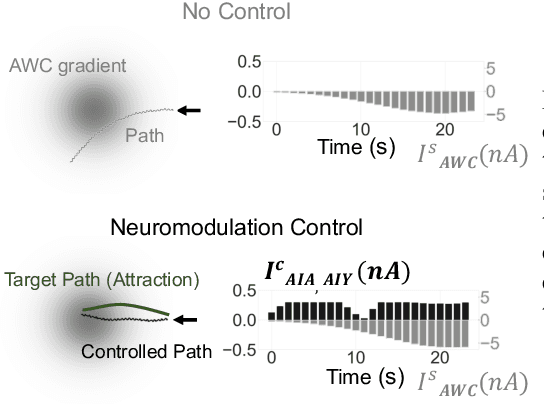
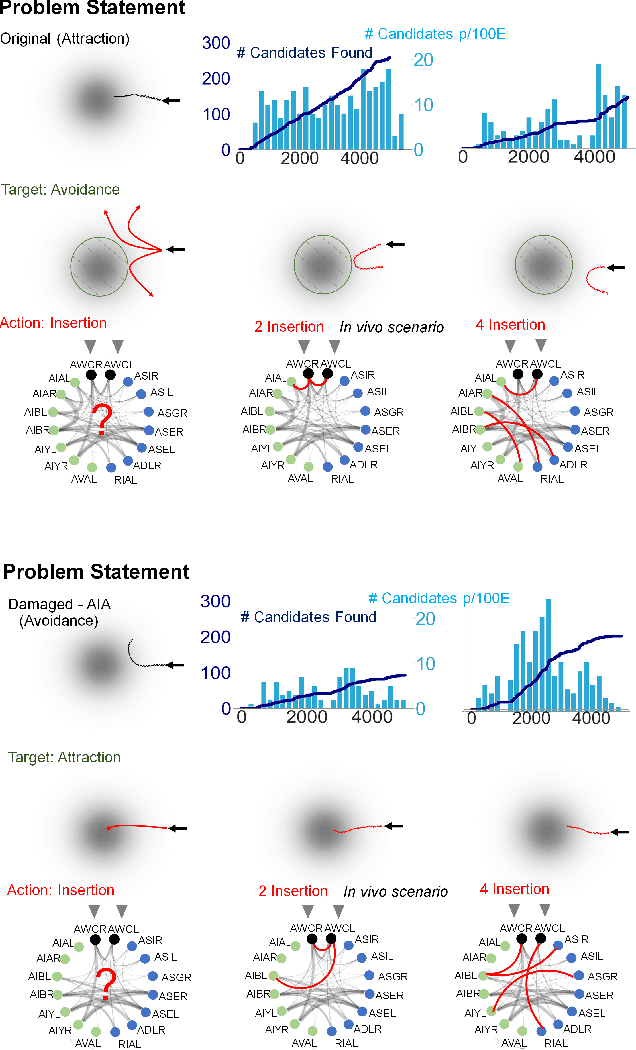
Abstract:We present a novel methodology for control of neural circuits based on deep reinforcement learning. Our approach achieves aimed behavior by generating external continuous stimulation of existing neural circuits (neuromodulation control) or modulations of neural circuits architecture (connectome control). Both forms of control are challenging due to nonlinear and recurrent complexity of neural activity. To infer candidate control policies, our approach maps neural circuits and their connectome into a grid-world like setting and infers the actions needed to achieve aimed behavior. The actions are inferred by adaptation of deep Q-learning methods known for their robust performance in navigating grid-worlds. We apply our approach to the model of \textit{C. elegans} which simulates the full somatic nervous system with muscles and body. Our framework successfully infers neuropeptidic currents and synaptic architectures for control of chemotaxis. Our findings are consistent with in vivo measurements and provide additional insights into neural control of chemotaxis. We further demonstrate the generality and scalability of our methods by inferring chemotactic neural circuits from scratch.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge