Li-Fang Cheng
Uncertainty-Aware PPG-2-ECG for Enhanced Cardiovascular Diagnosis using Diffusion Models
May 19, 2024Abstract:Analyzing the cardiovascular system condition via Electrocardiography (ECG) is a common and highly effective approach, and it has been practiced and perfected over many decades. ECG sensing is non-invasive and relatively easy to acquire, and yet it is still cumbersome for holter monitoring tests that may span over hours and even days. A possible alternative in this context is Photoplethysmography (PPG): An optically-based signal that measures blood volume fluctuations, as typically sensed by conventional ``wearable devices''. While PPG presents clear advantages in acquisition, convenience, and cost-effectiveness, ECG provides more comprehensive information, allowing for a more precise detection of heart conditions. This implies that a conversion from PPG to ECG, as recently discussed in the literature, inherently involves an unavoidable level of uncertainty. In this paper we introduce a novel methodology for addressing the PPG-2-ECG conversion, and offer an enhanced classification of cardiovascular conditions using the given PPG, all while taking into account the uncertainties arising from the conversion process. We provide a mathematical justification for our proposed computational approach, and present empirical studies demonstrating its superior performance compared to state-of-the-art baseline methods.
Patient-Specific Effects of Medication Using Latent Force Models with Gaussian Processes
Jun 01, 2019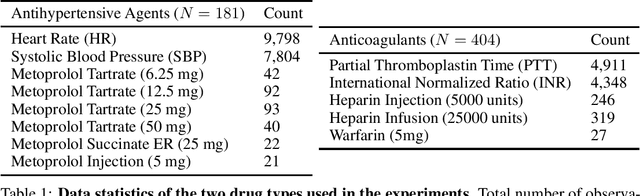
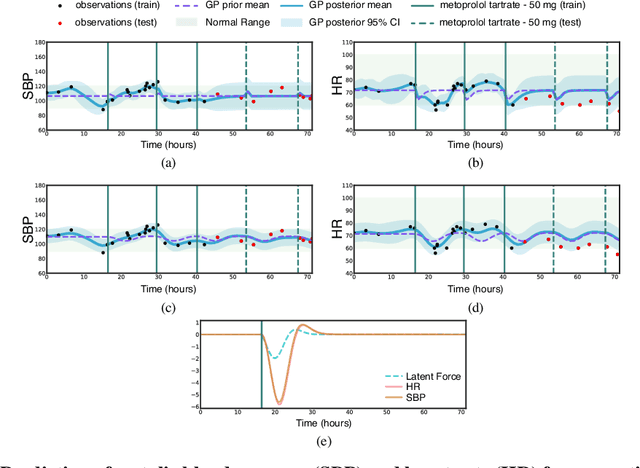
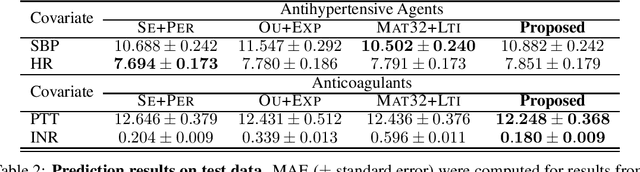
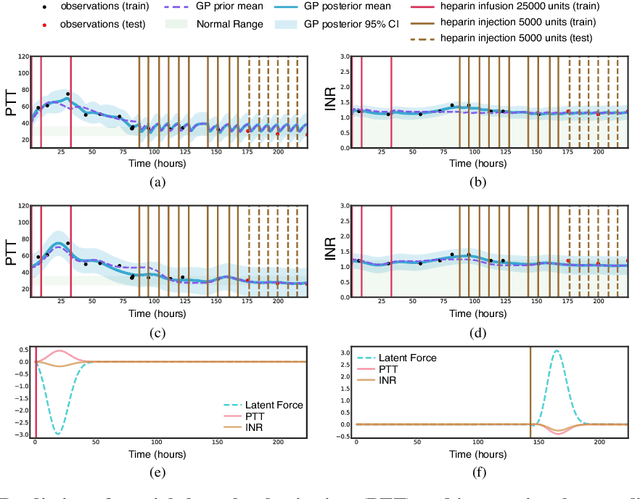
Abstract:Multi-output Gaussian processes (GPs) are a flexible Bayesian nonparametric framework that has proven useful in jointly modeling the physiological states of patients in medical time series data. However, capturing the short-term effects of drugs and therapeutic interventions on patient physiological state remains challenging. We propose a novel approach that models the effect of interventions as a hybrid Gaussian process composed of a GP capturing patient physiology convolved with a latent force model capturing effects of treatments on specific physiological features. This convolution of a multi-output GP with a GP including a causal time-marked kernel leads to a well-characterized model of the patients' physiological state responding to interventions. We show that our model leads to analytically tractable cross-covariance functions, allowing scalable inference. Our hierarchical model includes estimates of patient-specific effects but allows sharing of support across patients. Our approach achieves competitive predictive performance on challenging hospital data, where we recover patient-specific response to the administration of three common drugs: one antihypertensive drug and two anticoagulants.
An Optimal Policy for Patient Laboratory Tests in Intensive Care Units
Aug 14, 2018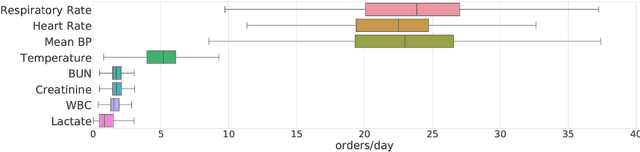
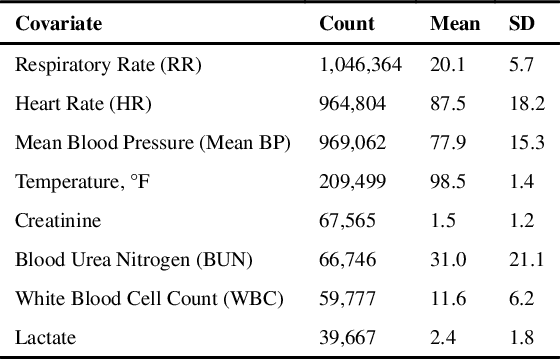
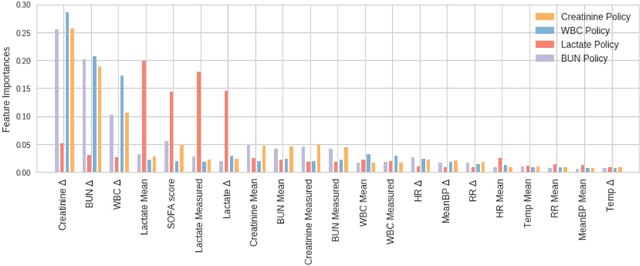
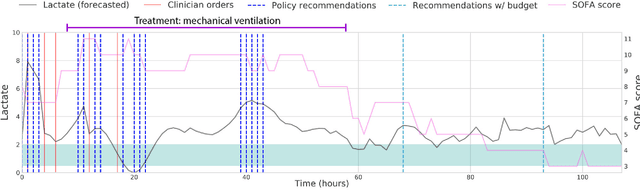
Abstract:Laboratory testing is an integral tool in the management of patient care in hospitals, particularly in intensive care units (ICUs). There exists an inherent trade-off in the selection and timing of lab tests between considerations of the expected utility in clinical decision-making of a given test at a specific time, and the associated cost or risk it poses to the patient. In this work, we introduce a framework that learns policies for ordering lab tests which optimizes for this trade-off. Our approach uses batch off-policy reinforcement learning with a composite reward function based on clinical imperatives, applied to data that include examples of clinicians ordering labs for patients. To this end, we develop and extend principles of Pareto optimality to improve the selection of actions based on multiple reward function components while respecting typical procedural considerations and prioritization of clinical goals in the ICU. Our experiments show that we can estimate a policy that reduces the frequency of lab tests and optimizes timing to minimize information redundancy. We also find that the estimated policies typically suggest ordering lab tests well ahead of critical onsets--such as mechanical ventilation or dialysis--that depend on the lab results. We evaluate our approach by quantifying how these policies may initiate earlier onset of treatment.
Sparse Multi-Output Gaussian Processes for Medical Time Series Prediction
Jun 21, 2018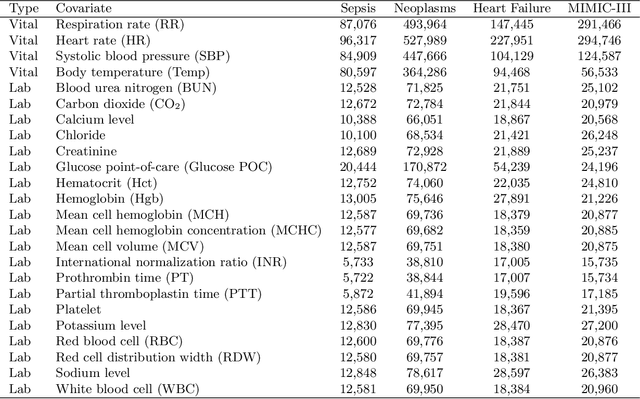
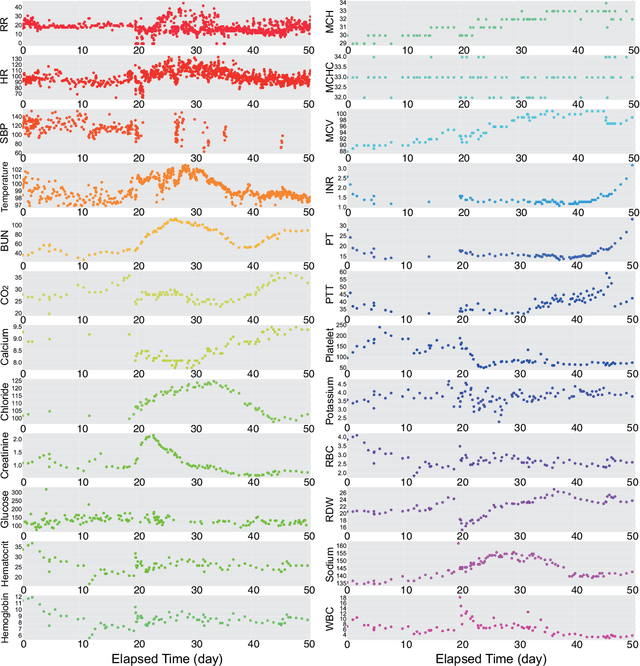
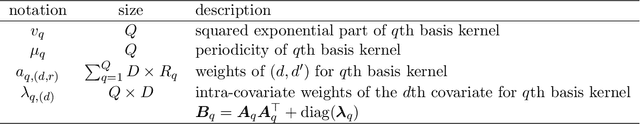
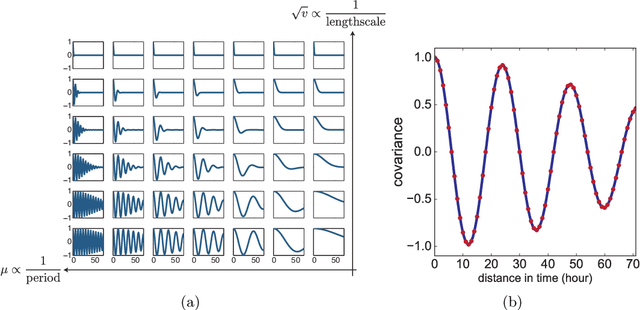
Abstract:In the scenario of real-time monitoring of hospital patients, high-quality inference of patients' health status using all information available from clinical covariates and lab tests is essential to enable successful medical interventions and improve patient outcomes. Developing a computational framework that can learn from observational large-scale electronic health records (EHRs) and make accurate real-time predictions is a critical step. In this work, we develop and explore a Bayesian nonparametric model based on Gaussian process (GP) regression for hospital patient monitoring. We propose MedGP, a statistical framework that incorporates 24 clinical and lab covariates and supports a rich reference data set from which relationships between observed covariates may be inferred and exploited for high-quality inference of patient state over time. To do this, we develop a highly structured sparse GP kernel to enable tractable computation over tens of thousands of time points while estimating correlations among clinical covariates, patients, and periodicity in patient observations. MedGP has a number of benefits over current methods, including (i) not requiring an alignment of the time series data, (ii) quantifying confidence regions in the predictions, (iii) exploiting a vast and rich database of patients, and (iv) inferring interpretable relationships among clinical covariates. We evaluate and compare results from MedGP on the task of online prediction for three patient subgroups from two medical data sets across 8,043 patients. We found MedGP improves online prediction over baseline methods for nearly all covariates across different disease subgroups and studies. The publicly available code is at https://github.com/bee-hive/MedGP.
Large Linear Multi-output Gaussian Process Learning
Oct 23, 2017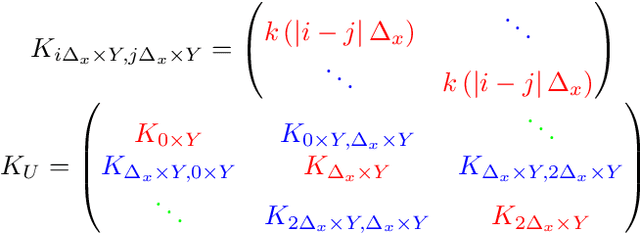
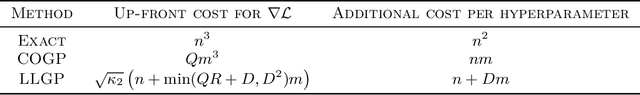
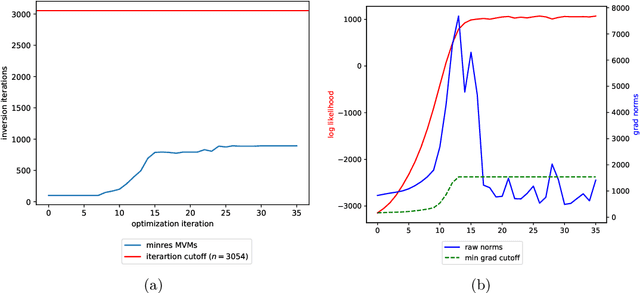
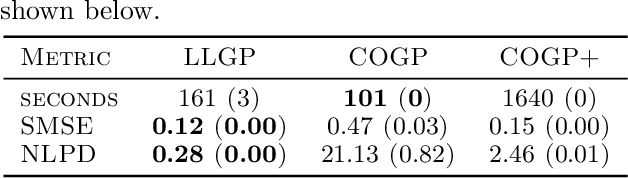
Abstract:Gaussian processes (GPs), or distributions over arbitrary functions in a continuous domain, can be generalized to the multi-output case: a linear model of coregionalization (LMC) is one approach. LMCs estimate and exploit correlations across the multiple outputs. While model estimation can be performed efficiently for single-output GPs, these assume stationarity, but in the multi-output case the cross-covariance interaction is not stationary. We propose Large Linear GP (LLGP), which circumvents the need for stationarity by inducing structure in the LMC kernel through a common grid of inputs shared between outputs, enabling optimization of GP hyperparameters for multi-dimensional outputs and low-dimensional inputs. When applied to synthetic two-dimensional and real time series data, we find our theoretical improvement relative to the current solutions for multi-output GPs is realized with LLGP reducing training time while improving or maintaining predictive mean accuracy. Moreover, by using a direct likelihood approximation rather than a variational one, model confidence estimates are significantly improved.
A Reinforcement Learning Approach to Weaning of Mechanical Ventilation in Intensive Care Units
Apr 20, 2017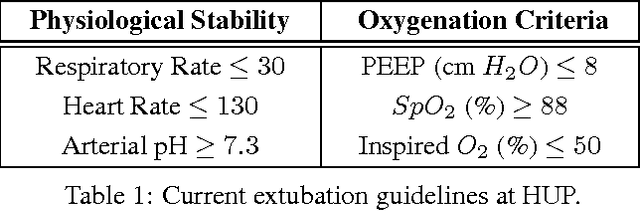
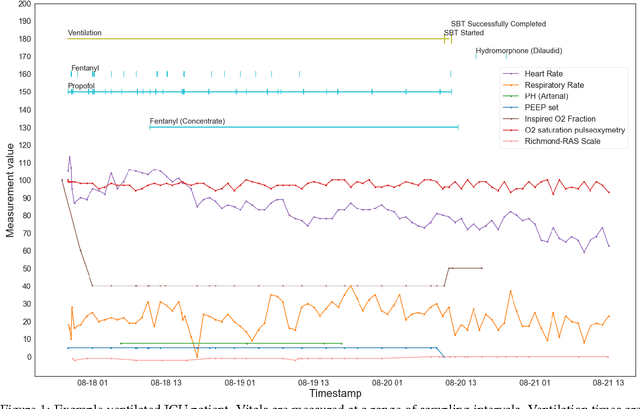
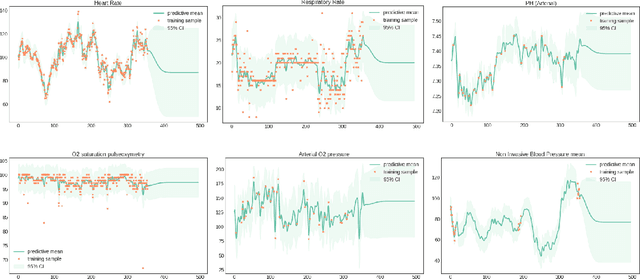
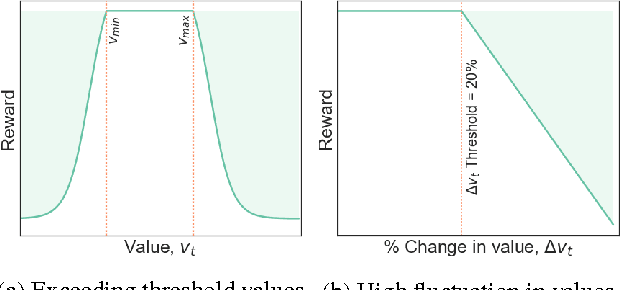
Abstract:The management of invasive mechanical ventilation, and the regulation of sedation and analgesia during ventilation, constitutes a major part of the care of patients admitted to intensive care units. Both prolonged dependence on mechanical ventilation and premature extubation are associated with increased risk of complications and higher hospital costs, but clinical opinion on the best protocol for weaning patients off of a ventilator varies. This work aims to develop a decision support tool that uses available patient information to predict time-to-extubation readiness and to recommend a personalized regime of sedation dosage and ventilator support. To this end, we use off-policy reinforcement learning algorithms to determine the best action at a given patient state from sub-optimal historical ICU data. We compare treatment policies from fitted Q-iteration with extremely randomized trees and with feedforward neural networks, and demonstrate that the policies learnt show promise in recommending weaning protocols with improved outcomes, in terms of minimizing rates of reintubation and regulating physiological stability.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge