Evangelos B. Mazomenos
Learning from Single Timestamps: Complexity Estimation in Laparoscopic Cholecystectomy
Nov 06, 2025Abstract:Purpose: Accurate assessment of surgical complexity is essential in Laparoscopic Cholecystectomy (LC), where severe inflammation is associated with longer operative times and increased risk of postoperative complications. The Parkland Grading Scale (PGS) provides a clinically validated framework for stratifying inflammation severity; however, its automation in surgical videos remains largely unexplored, particularly in realistic scenarios where complete videos must be analyzed without prior manual curation. Methods: In this work, we introduce STC-Net, a novel framework for SingleTimestamp-based Complexity estimation in LC via the PGS, designed to operate under weak temporal supervision. Unlike prior methods limited to static images or manually trimmed clips, STC-Net operates directly on full videos. It jointly performs temporal localization and grading through a localization, window proposal, and grading module. We introduce a novel loss formulation combining hard and soft localization objectives and background-aware grading supervision. Results: Evaluated on a private dataset of 1,859 LC videos, STC-Net achieves an accuracy of 62.11% and an F1-score of 61.42%, outperforming non-localized baselines by over 10% in both metrics and highlighting the effectiveness of weak supervision for surgical complexity assessment. Conclusion: STC-Net demonstrates a scalable and effective approach for automated PGS-based surgical complexity estimation from full LC videos, making it promising for post-operative analysis and surgical training.
Exploring Pre-training Across Domains for Few-Shot Surgical Skill Assessment
Sep 11, 2025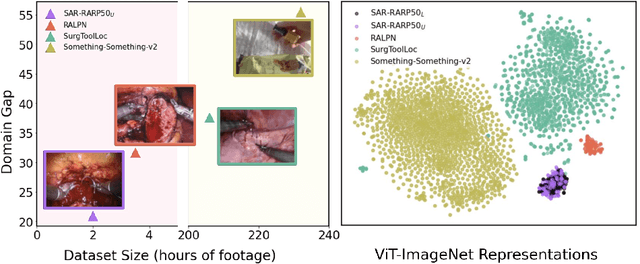

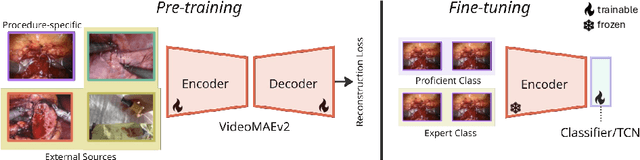
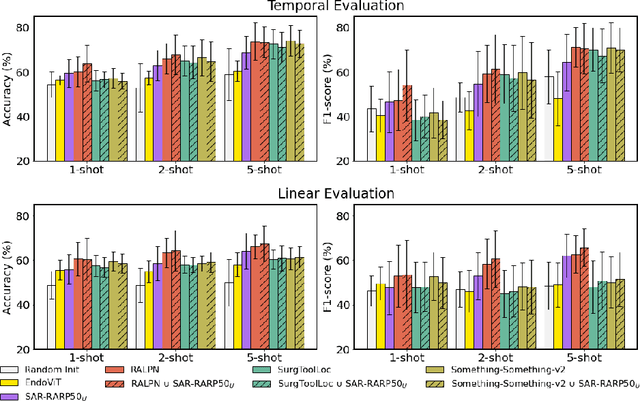
Abstract:Automated surgical skill assessment (SSA) is a central task in surgical computer vision. Developing robust SSA models is challenging due to the scarcity of skill annotations, which are time-consuming to produce and require expert consensus. Few-shot learning (FSL) offers a scalable alternative enabling model development with minimal supervision, though its success critically depends on effective pre-training. While widely studied for several surgical downstream tasks, pre-training has remained largely unexplored in SSA. In this work, we formulate SSA as a few-shot task and investigate how self-supervised pre-training strategies affect downstream few-shot SSA performance. We annotate a publicly available robotic surgery dataset with Objective Structured Assessment of Technical Skill (OSATS) scores, and evaluate various pre-training sources across three few-shot settings. We quantify domain similarity and analyze how domain gap and the inclusion of procedure-specific data into pre-training influence transferability. Our results show that small but domain-relevant datasets can outperform large scale, less aligned ones, achieving accuracies of 60.16%, 66.03%, and 73.65% in the 1-, 2-, and 5-shot settings, respectively. Moreover, incorporating procedure-specific data into pre-training with a domain-relevant external dataset significantly boosts downstream performance, with an average gain of +1.22% in accuracy and +2.28% in F1-score; however, applying the same strategy with less similar but large-scale sources can instead lead to performance degradation. Code and models are available at https://github.com/anastadimi/ssa-fsl.
StereoMamba: Real-time and Robust Intraoperative Stereo Disparity Estimation via Long-range Spatial Dependencies
Apr 24, 2025Abstract:Stereo disparity estimation is crucial for obtaining depth information in robot-assisted minimally invasive surgery (RAMIS). While current deep learning methods have made significant advancements, challenges remain in achieving an optimal balance between accuracy, robustness, and inference speed. To address these challenges, we propose the StereoMamba architecture, which is specifically designed for stereo disparity estimation in RAMIS. Our approach is based on a novel Feature Extraction Mamba (FE-Mamba) module, which enhances long-range spatial dependencies both within and across stereo images. To effectively integrate multi-scale features from FE-Mamba, we then introduce a novel Multidimensional Feature Fusion (MFF) module. Experiments against the state-of-the-art on the ex-vivo SCARED benchmark demonstrate that StereoMamba achieves superior performance on EPE of 2.64 px and depth MAE of 2.55 mm, the second-best performance on Bad2 of 41.49% and Bad3 of 26.99%, while maintaining an inference speed of 21.28 FPS for a pair of high-resolution images (1280*1024), striking the optimum balance between accuracy, robustness, and efficiency. Furthermore, by comparing synthesized right images, generated from warping left images using the generated disparity maps, with the actual right image, StereoMamba achieves the best average SSIM (0.8970) and PSNR (16.0761), exhibiting strong zero-shot generalization on the in-vivo RIS2017 and StereoMIS datasets.
EndoLRMGS: Complete Endoscopic Scene Reconstruction combining Large Reconstruction Modelling and Gaussian Splatting
Mar 28, 2025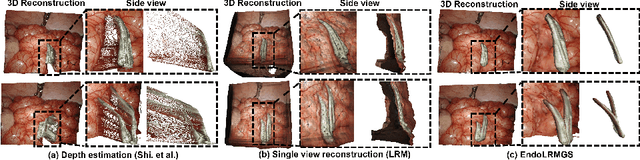
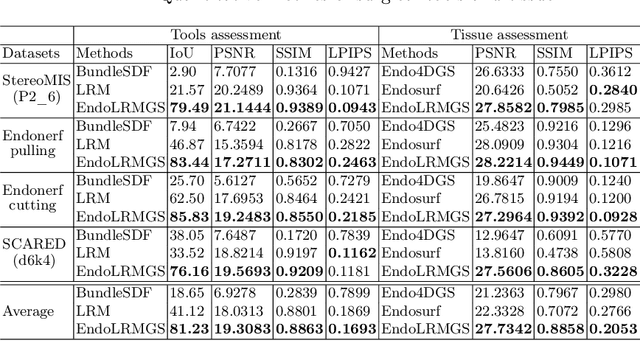
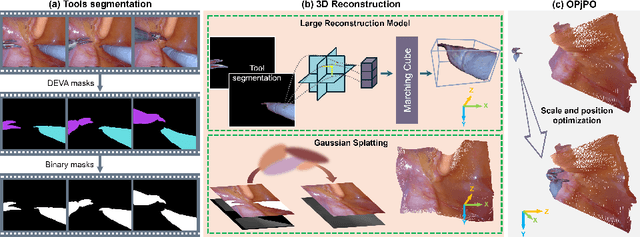
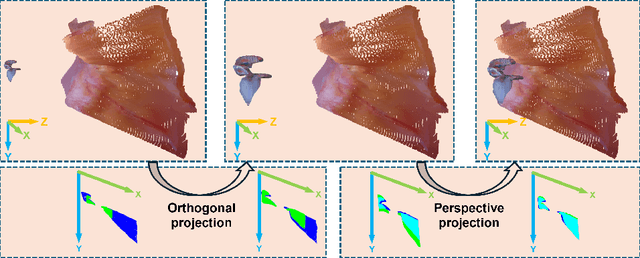
Abstract:Complete reconstruction of surgical scenes is crucial for robot-assisted surgery (RAS). Deep depth estimation is promising but existing works struggle with depth discontinuities, resulting in noisy predictions at object boundaries and do not achieve complete reconstruction omitting occluded surfaces. To address these issues we propose EndoLRMGS, that combines Large Reconstruction Modelling (LRM) and Gaussian Splatting (GS), for complete surgical scene reconstruction. GS reconstructs deformable tissues and LRM generates 3D models for surgical tools while position and scale are subsequently optimized by introducing orthogonal perspective joint projection optimization (OPjPO) to enhance accuracy. In experiments on four surgical videos from three public datasets, our method improves the Intersection-over-union (IoU) of tool 3D models in 2D projections by>40%. Additionally, EndoLRMGS improves the PSNR of the tools projection from 3.82% to 11.07%. Tissue rendering quality also improves, with PSNR increasing from 0.46% to 49.87%, and SSIM from 1.53% to 29.21% across all test videos.
3D Acetabular Surface Reconstruction from 2D Pre-operative X-ray Images using SRVF Elastic Registration and Deformation Graph
Mar 28, 2025Abstract:Accurate and reliable selection of the appropriate acetabular cup size is crucial for restoring joint biomechanics in total hip arthroplasty (THA). This paper proposes a novel framework that integrates square-root velocity function (SRVF)-based elastic shape registration technique with an embedded deformation (ED) graph approach to reconstruct the 3D articular surface of the acetabulum by fusing multiple views of 2D pre-operative pelvic X-ray images and a hemispherical surface model. The SRVF-based elastic registration establishes 2D-3D correspondences between the parametric hemispherical model and X-ray images, and the ED framework incorporates the SRVF-derived correspondences as constraints to optimize the 3D acetabular surface reconstruction using nonlinear least-squares optimization. Validations using both simulation and real patient datasets are performed to demonstrate the robustness and the potential clinical value of the proposed algorithm. The reconstruction result can assist surgeons in selecting the correct acetabular cup on the first attempt in primary THA, minimising the need for revision surgery.
KEVS: Enhancing Segmentation of Visceral Adipose Tissue in Pre-Cystectomy CT with Gaussian Kernel Density Estimation
Mar 28, 2025Abstract:Purpose: The distribution of visceral adipose tissue (VAT) in cystectomy patients is indicative of the incidence of post-operative complications. Existing VAT segmentation methods for computed tomography (CT) employing intensity thresholding have limitations relating to inter-observer variability. Moreover, the difficulty in creating ground-truth masks limits the development of deep learning (DL) models for this task. This paper introduces a novel method for VAT prediction in pre-cystectomy CT, which is fully automated and does not require ground-truth VAT masks for training, overcoming aforementioned limitations. Methods: We introduce the Kernel density Enhanced VAT Segmentator ( KEVS), combining a DL semantic segmentation model, for multi-body feature prediction, with Gaussian kernel density estimation analysis of predicted subcutaneous adipose tissue to achieve accurate scan-specific predictions of VAT in the abdominal cavity. Uniquely for a DL pipeline, KEVS does not require ground-truth VAT masks. Results: We verify the ability of KEVS to accurately segment abdominal organs in unseen CT data and compare KEVS VAT segmentation predictions to existing state-of-the-art (SOTA) approaches in a dataset of 20 pre-cystectomy CT scans, collected from University College London Hospital (UCLH-Cyst), with expert ground-truth annotations. KEVS presents a 4.80% and 6.02% improvement in Dice Coefficient over the second best DL and thresholding-based VAT segmentation techniques respectively when evaluated on UCLH-Cyst. Conclusion: This research introduces KEVS; an automated, SOTA method for the prediction of VAT in pre-cystectomy CT which eliminates inter-observer variability and is trained entirely on open-source CT datasets which do not contain ground-truth VAT masks.
SurgRAW: Multi-Agent Workflow with Chain-of-Thought Reasoning for Surgical Intelligence
Mar 13, 2025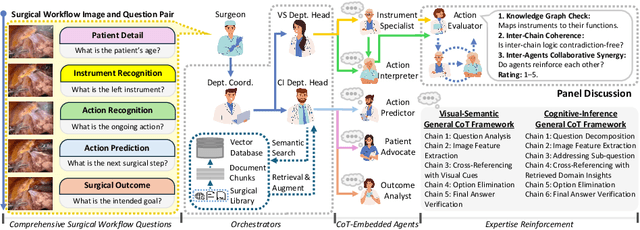
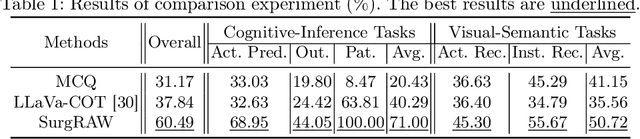
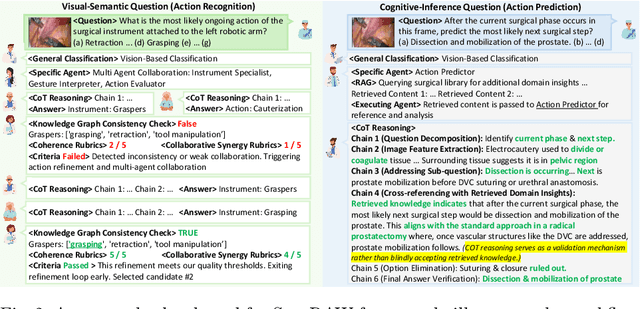
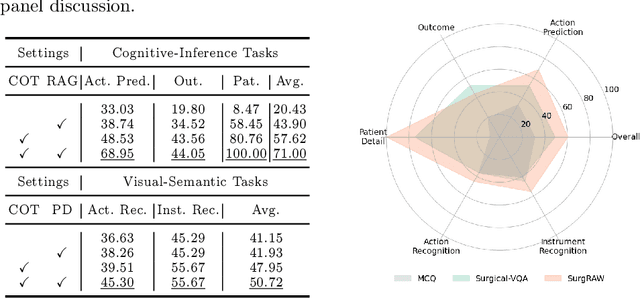
Abstract:Integration of Vision-Language Models (VLMs) in surgical intelligence is hindered by hallucinations, domain knowledge gaps, and limited understanding of task interdependencies within surgical scenes, undermining clinical reliability. While recent VLMs demonstrate strong general reasoning and thinking capabilities, they still lack the domain expertise and task-awareness required for precise surgical scene interpretation. Although Chain-of-Thought (CoT) can structure reasoning more effectively, current approaches rely on self-generated CoT steps, which often exacerbate inherent domain gaps and hallucinations. To overcome this, we present SurgRAW, a CoT-driven multi-agent framework that delivers transparent, interpretable insights for most tasks in robotic-assisted surgery. By employing specialized CoT prompts across five tasks: instrument recognition, action recognition, action prediction, patient data extraction, and outcome assessment, SurgRAW mitigates hallucinations through structured, domain-aware reasoning. Retrieval-Augmented Generation (RAG) is also integrated to external medical knowledge to bridge domain gaps and improve response reliability. Most importantly, a hierarchical agentic system ensures that CoT-embedded VLM agents collaborate effectively while understanding task interdependencies, with a panel discussion mechanism promotes logical consistency. To evaluate our method, we introduce SurgCoTBench, the first reasoning-based dataset with structured frame-level annotations. With comprehensive experiments, we demonstrate the effectiveness of proposed SurgRAW with 29.32% accuracy improvement over baseline VLMs on 12 robotic procedures, achieving the state-of-the-art performance and advancing explainable, trustworthy, and autonomous surgical assistance.
Distilling Knowledge into Quantum Vision Transformers for Biomedical Image Classification
Mar 10, 2025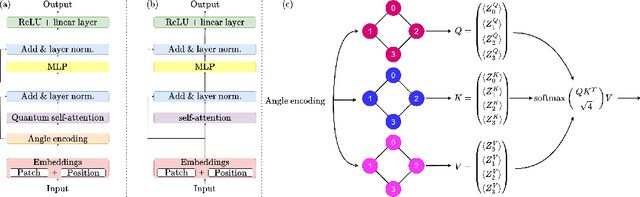
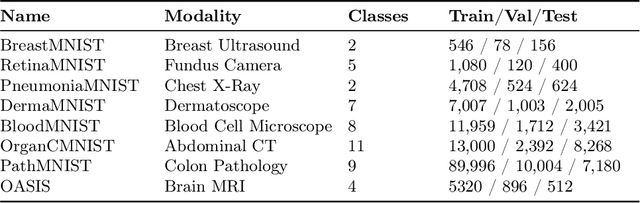
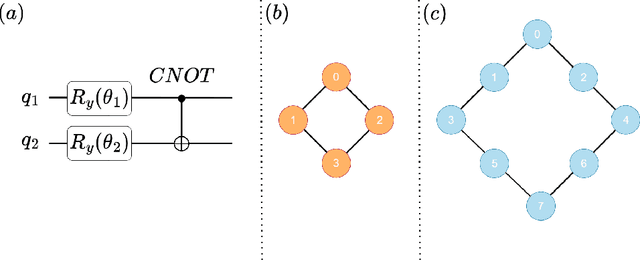
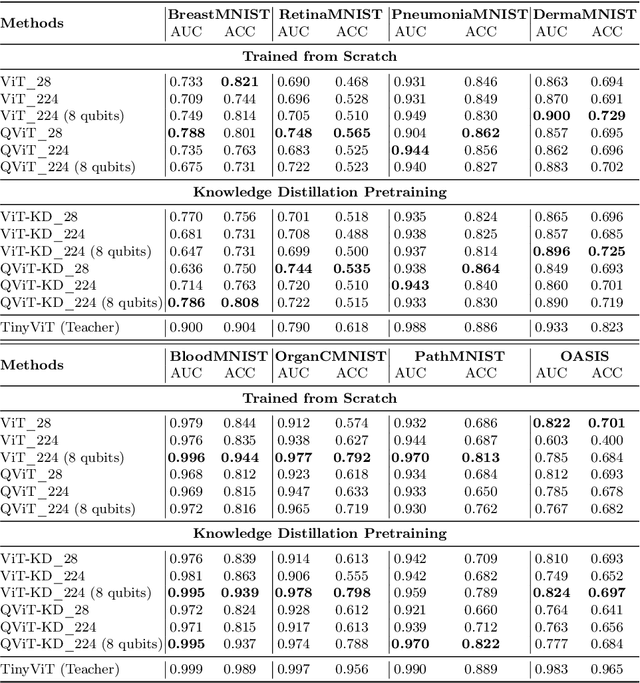
Abstract:Quantum vision transformers (QViTs) build on vision transformers (ViTs) by replacing linear layers within the self-attention mechanism with parameterised quantum neural networks (QNNs), harnessing quantum mechanical properties to improve feature representation. This hybrid approach aims to achieve superior performance, with significantly reduced model complexity as a result of the enriched feature representation, requiring fewer parameters. This paper proposes a novel QViT model for biomedical image classification and investigates its performance against comparable ViTs across eight diverse datasets, encompassing various modalities and classification tasks. We assess models trained from scratch and those pre-trained using knowledge distillation (KD) from high-quality teacher models. Our findings demonstrate that QViTs outperform comparable ViTs with average ROC AUC (0.863 vs 0.846) and accuracy (0.710 vs 0.687) when trained from scratch, and even compete with state-of-the-art classical models in multiple tasks, whilst being significantly more efficient (89% reduction in GFLOPs and 99.99% in parameter number). Additionally, we find that QViTs and ViTs respond equally well to KD, with QViT pre-training performance scaling with model complexity. This is the first investigation into the efficacy of deploying QViTs with KD for computer-aided diagnosis. Our results highlight the enormous potential of quantum machine learning (QML) in biomedical image analysis.
PitVQA++: Vector Matrix-Low-Rank Adaptation for Open-Ended Visual Question Answering in Pituitary Surgery
Feb 19, 2025Abstract:Vision-Language Models (VLMs) in visual question answering (VQA) offer a unique opportunity to enhance intra-operative decision-making, promote intuitive interactions, and significantly advancing surgical education. However, the development of VLMs for surgical VQA is challenging due to limited datasets and the risk of overfitting and catastrophic forgetting during full fine-tuning of pretrained weights. While parameter-efficient techniques like Low-Rank Adaptation (LoRA) and Matrix of Rank Adaptation (MoRA) address adaptation challenges, their uniform parameter distribution overlooks the feature hierarchy in deep networks, where earlier layers, that learn general features, require more parameters than later ones. This work introduces PitVQA++ with an open-ended PitVQA dataset and vector matrix-low-rank adaptation (Vector-MoLoRA), an innovative VLM fine-tuning approach for adapting GPT-2 to pituitary surgery. Open-Ended PitVQA comprises around 101,803 frames from 25 procedural videos with 745,972 question-answer sentence pairs, covering key surgical elements such as phase and step recognition, context understanding, tool detection, localization, and interactions recognition. Vector-MoLoRA incorporates the principles of LoRA and MoRA to develop a matrix-low-rank adaptation strategy that employs vector ranking to allocate more parameters to earlier layers, gradually reducing them in the later layers. Our approach, validated on the Open-Ended PitVQA and EndoVis18-VQA datasets, effectively mitigates catastrophic forgetting while significantly enhancing performance over recent baselines. Furthermore, our risk-coverage analysis highlights its enhanced reliability and trustworthiness in handling uncertain predictions. Our source code and dataset is available at~\url{https://github.com/HRL-Mike/PitVQA-Plus}.
Personalizing Federated Instrument Segmentation with Visual Trait Priors in Robotic Surgery
Aug 06, 2024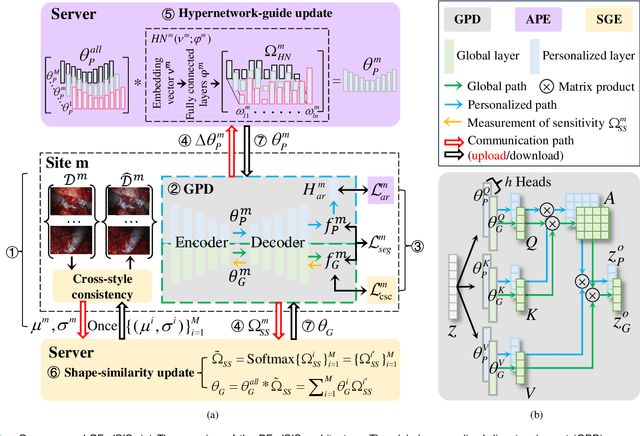
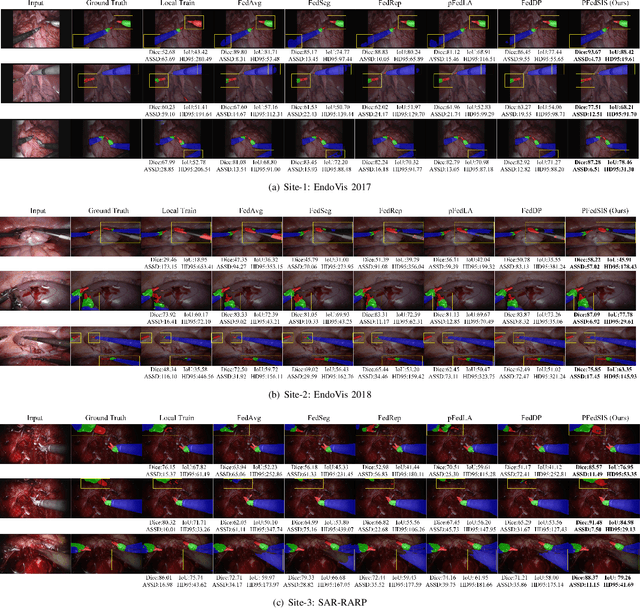
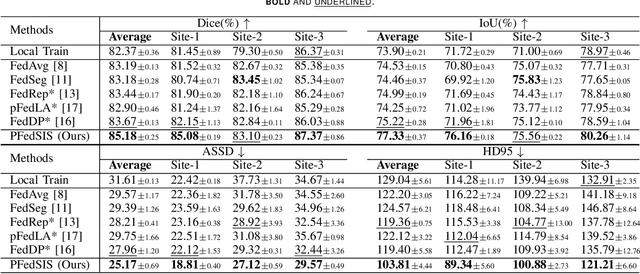

Abstract:Personalized federated learning (PFL) for surgical instrument segmentation (SIS) is a promising approach. It enables multiple clinical sites to collaboratively train a series of models in privacy, with each model tailored to the individual distribution of each site. Existing PFL methods rarely consider the personalization of multi-headed self-attention, and do not account for appearance diversity and instrument shape similarity, both inherent in surgical scenes. We thus propose PFedSIS, a novel PFL method with visual trait priors for SIS, incorporating global-personalized disentanglement (GPD), appearance-regulation personalized enhancement (APE), and shape-similarity global enhancement (SGE), to boost SIS performance in each site. GPD represents the first attempt at head-wise assignment for multi-headed self-attention personalization. To preserve the unique appearance representation of each site and gradually leverage the inter-site difference, APE introduces appearance regulation and provides customized layer-wise aggregation solutions via hypernetworks for each site's personalized parameters. The mutual shape information of instruments is maintained and shared via SGE, which enhances the cross-style shape consistency on the image level and computes the shape-similarity contribution of each site on the prediction level for updating the global parameters. PFedSIS outperforms state-of-the-art methods with +1.51% Dice, +2.11% IoU, -2.79 ASSD, -15.55 HD95 performance gains. The corresponding code and models will be released at https://github.com/wzjialang/PFedSIS.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge