Dmitriy Dligach
Evaluating Retrieval-Augmented Generation vs. Long-Context Input for Clinical Reasoning over EHRs
Aug 20, 2025Abstract:Electronic health records (EHRs) are long, noisy, and often redundant, posing a major challenge for the clinicians who must navigate them. Large language models (LLMs) offer a promising solution for extracting and reasoning over this unstructured text, but the length of clinical notes often exceeds even state-of-the-art models' extended context windows. Retrieval-augmented generation (RAG) offers an alternative by retrieving task-relevant passages from across the entire EHR, potentially reducing the amount of required input tokens. In this work, we propose three clinical tasks designed to be replicable across health systems with minimal effort: 1) extracting imaging procedures, 2) generating timelines of antibiotic use, and 3) identifying key diagnoses. Using EHRs from actual hospitalized patients, we test three state-of-the-art LLMs with varying amounts of provided context, using either targeted text retrieval or the most recent clinical notes. We find that RAG closely matches or exceeds the performance of using recent notes, and approaches the performance of using the models' full context while requiring drastically fewer input tokens. Our results suggest that RAG remains a competitive and efficient approach even as newer models become capable of handling increasingly longer amounts of text.
The use of large language models to enhance cancer clinical trial educational materials
Dec 02, 2024Abstract:Cancer clinical trials often face challenges in recruitment and engagement due to a lack of participant-facing informational and educational resources. This study investigated the potential of Large Language Models (LLMs), specifically GPT4, in generating patient-friendly educational content from clinical trial informed consent forms. Using data from ClinicalTrials.gov, we employed zero-shot learning for creating trial summaries and one-shot learning for developing multiple-choice questions, evaluating their effectiveness through patient surveys and crowdsourced annotation. Results showed that GPT4-generated summaries were both readable and comprehensive, and may improve patients' understanding and interest in clinical trials. The multiple-choice questions demonstrated high accuracy and agreement with crowdsourced annotators. For both resource types, hallucinations were identified that require ongoing human oversight. The findings demonstrate the potential of LLMs "out-of-the-box" to support the generation of clinical trial education materials with minimal trial-specific engineering, but implementation with a human-in-the-loop is still needed to avoid misinformation risks.
Position Paper On Diagnostic Uncertainty Estimation from Large Language Models: Next-Word Probability Is Not Pre-test Probability
Nov 07, 2024Abstract:Large language models (LLMs) are being explored for diagnostic decision support, yet their ability to estimate pre-test probabilities, vital for clinical decision-making, remains limited. This study evaluates two LLMs, Mistral-7B and Llama3-70B, using structured electronic health record data on three diagnosis tasks. We examined three current methods of extracting LLM probability estimations and revealed their limitations. We aim to highlight the need for improved techniques in LLM confidence estimation.
When Raw Data Prevails: Are Large Language Model Embeddings Effective in Numerical Data Representation for Medical Machine Learning Applications?
Aug 15, 2024



Abstract:The introduction of Large Language Models (LLMs) has advanced data representation and analysis, bringing significant progress in their use for medical questions and answering. Despite these advancements, integrating tabular data, especially numerical data pivotal in clinical contexts, into LLM paradigms has not been thoroughly explored. In this study, we examine the effectiveness of vector representations from last hidden states of LLMs for medical diagnostics and prognostics using electronic health record (EHR) data. We compare the performance of these embeddings with that of raw numerical EHR data when used as feature inputs to traditional machine learning (ML) algorithms that excel at tabular data learning, such as eXtreme Gradient Boosting. We focus on instruction-tuned LLMs in a zero-shot setting to represent abnormal physiological data and evaluating their utilities as feature extractors to enhance ML classifiers for predicting diagnoses, length of stay, and mortality. Furthermore, we examine prompt engineering techniques on zero-shot and few-shot LLM embeddings to measure their impact comprehensively. Although findings suggest the raw data features still prevails in medical ML tasks, zero-shot LLM embeddings demonstrate competitive results, suggesting a promising avenue for future research in medical applications.
Improving Clinical NLP Performance through Language Model-Generated Synthetic Clinical Data
Mar 28, 2024
Abstract:Generative models have been showing potential for producing data in mass. This study explores the enhancement of clinical natural language processing performance by utilizing synthetic data generated from advanced language models. Promising results show feasible applications in such a high-stakes domain.
Leveraging A Medical Knowledge Graph into Large Language Models for Diagnosis Prediction
Aug 28, 2023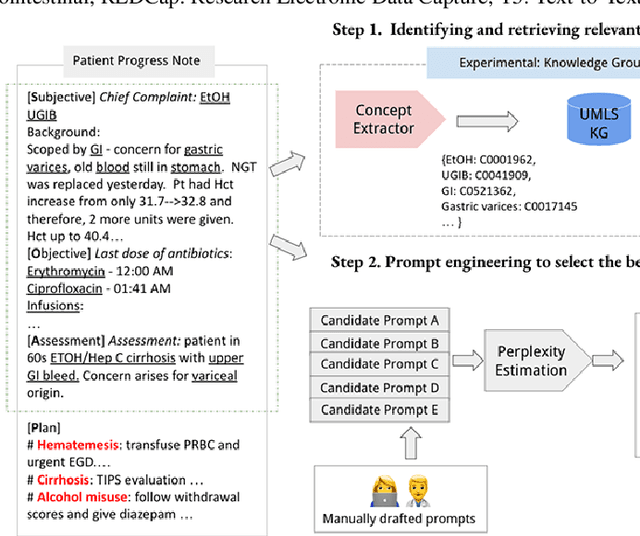

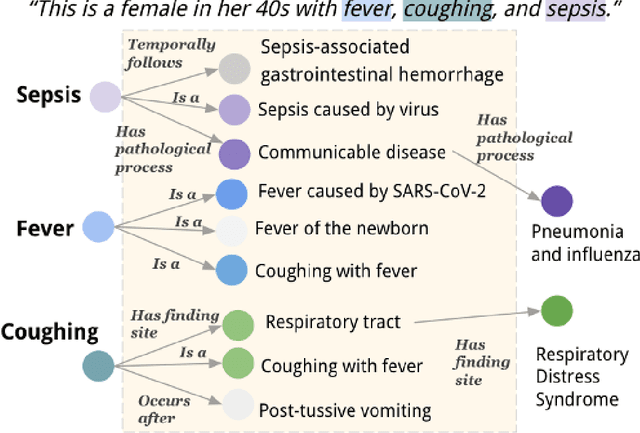
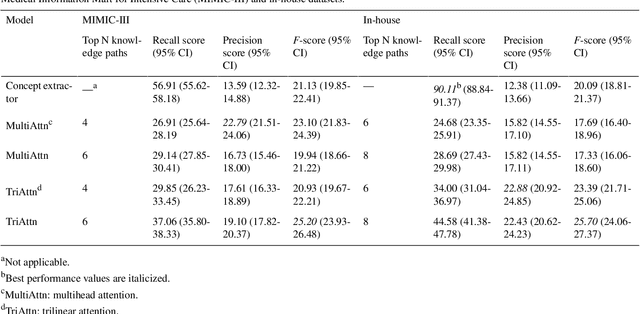
Abstract:Electronic Health Records (EHRs) and routine documentation practices play a vital role in patients' daily care, providing a holistic record of health, diagnoses, and treatment. However, complex and verbose EHR narratives overload healthcare providers, risking diagnostic inaccuracies. While Large Language Models (LLMs) have showcased their potential in diverse language tasks, their application in the healthcare arena needs to ensure the minimization of diagnostic errors and the prevention of patient harm. In this paper, we outline an innovative approach for augmenting the proficiency of LLMs in the realm of automated diagnosis generation, achieved through the incorporation of a medical knowledge graph (KG) and a novel graph model: Dr.Knows, inspired by the clinical diagnostic reasoning process. We derive the KG from the National Library of Medicine's Unified Medical Language System (UMLS), a robust repository of biomedical knowledge. Our method negates the need for pre-training and instead leverages the KG as an auxiliary instrument aiding in the interpretation and summarization of complex medical concepts. Using real-world hospital datasets, our experimental results demonstrate that the proposed approach of combining LLMs with KG has the potential to improve the accuracy of automated diagnosis generation. More importantly, our approach offers an explainable diagnostic pathway, edging us closer to the realization of AI-augmented diagnostic decision support systems.
Multi-Task Training with In-Domain Language Models for Diagnostic Reasoning
Jun 13, 2023Abstract:Generative artificial intelligence (AI) is a promising direction for augmenting clinical diagnostic decision support and reducing diagnostic errors, a leading contributor to medical errors. To further the development of clinical AI systems, the Diagnostic Reasoning Benchmark (DR.BENCH) was introduced as a comprehensive generative AI framework, comprised of six tasks representing key components in clinical reasoning. We present a comparative analysis of in-domain versus out-of-domain language models as well as multi-task versus single task training with a focus on the problem summarization task in DR.BENCH (Gao et al., 2023). We demonstrate that a multi-task, clinically trained language model outperforms its general domain counterpart by a large margin, establishing a new state-of-the-art performance, with a ROUGE-L score of 28.55. This research underscores the value of domain-specific training for optimizing clinical diagnostic reasoning tasks.
Overview of the Problem List Summarization (ProbSum) 2023 Shared Task on Summarizing Patients' Active Diagnoses and Problems from Electronic Health Record Progress Notes
Jun 08, 2023Abstract:The BioNLP Workshop 2023 initiated the launch of a shared task on Problem List Summarization (ProbSum) in January 2023. The aim of this shared task is to attract future research efforts in building NLP models for real-world diagnostic decision support applications, where a system generating relevant and accurate diagnoses will augment the healthcare providers decision-making process and improve the quality of care for patients. The goal for participants is to develop models that generated a list of diagnoses and problems using input from the daily care notes collected from the hospitalization of critically ill patients. Eight teams submitted their final systems to the shared task leaderboard. In this paper, we describe the tasks, datasets, evaluation metrics, and baseline systems. Additionally, the techniques and results of the evaluation of the different approaches tried by the participating teams are summarized.
Progress Note Understanding -- Assessment and Plan Reasoning: Overview of the 2022 N2C2 Track 3 Shared Task
Mar 14, 2023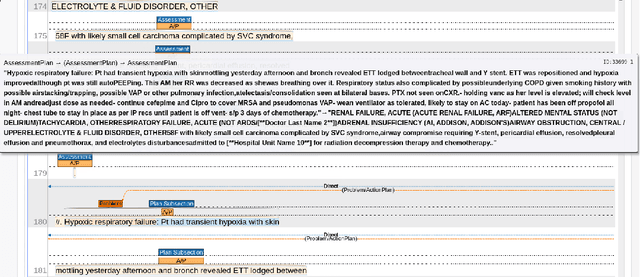
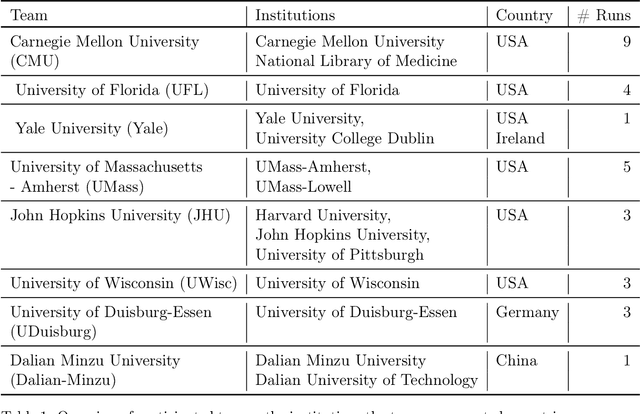
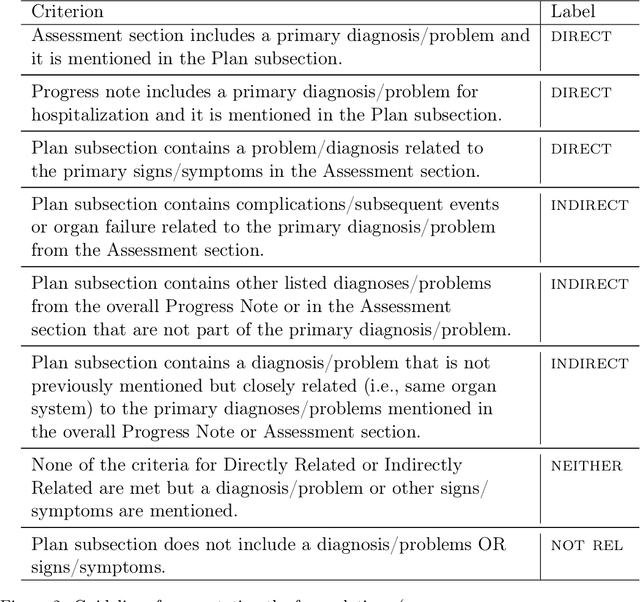
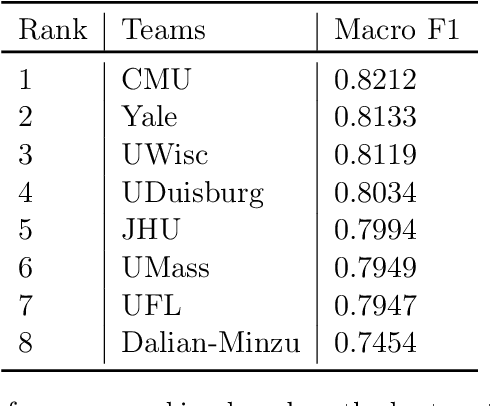
Abstract:Daily progress notes are common types in the electronic health record (EHR) where healthcare providers document the patient's daily progress and treatment plans. The EHR is designed to document all the care provided to patients, but it also enables note bloat with extraneous information that distracts from the diagnoses and treatment plans. Applications of natural language processing (NLP) in the EHR is a growing field with the majority of methods in information extraction. Few tasks use NLP methods for downstream diagnostic decision support. We introduced the 2022 National NLP Clinical Challenge (N2C2) Track 3: Progress Note Understanding - Assessment and Plan Reasoning as one step towards a new suite of tasks. The Assessment and Plan Reasoning task focuses on the most critical components of progress notes, Assessment and Plan subsections where health problems and diagnoses are contained. The goal of the task was to develop and evaluate NLP systems that automatically predict causal relations between the overall status of the patient contained in the Assessment section and its relation to each component of the Plan section which contains the diagnoses and treatment plans. The goal of the task was to identify and prioritize diagnoses as the first steps in diagnostic decision support to find the most relevant information in long documents like daily progress notes. We present the results of 2022 n2c2 Track 3 and provide a description of the data, evaluation, participation and system performance.
DR.BENCH: Diagnostic Reasoning Benchmark for Clinical Natural Language Processing
Sep 29, 2022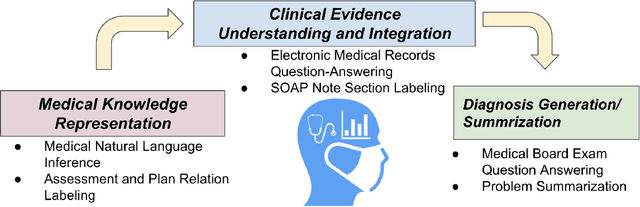
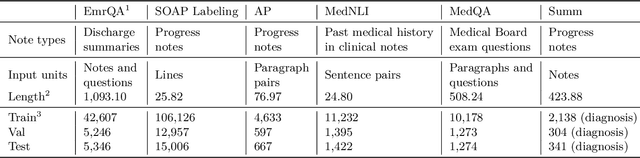
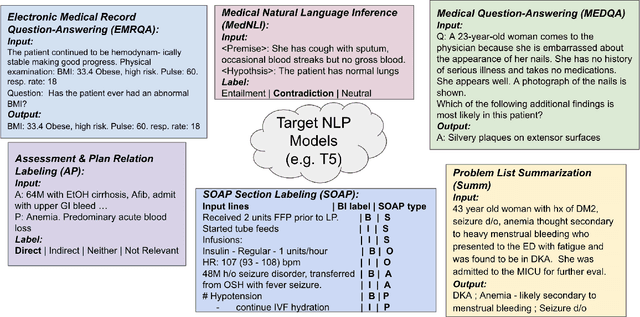
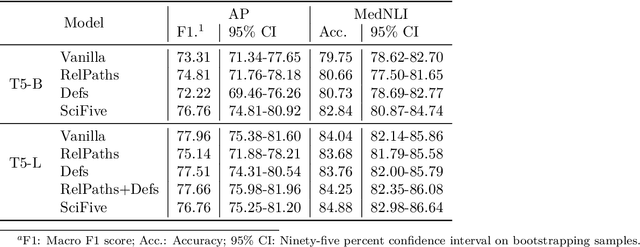
Abstract:The meaningful use of electronic health records (EHR) continues to progress in the digital era with clinical decision support systems augmented by artificial intelligence. A priority in improving provider experience is to overcome information overload and reduce the cognitive burden so fewer medical errors and cognitive biases are introduced during patient care. One major type of medical error is diagnostic error due to systematic or predictable errors in judgment that rely on heuristics. The potential for clinical natural language processing (cNLP) to model diagnostic reasoning in humans with forward reasoning from data to diagnosis and potentially reduce the cognitive burden and medical error has not been investigated. Existing tasks to advance the science in cNLP have largely focused on information extraction and named entity recognition through classification tasks. We introduce a novel suite of tasks coined as Diagnostic Reasoning Benchmarks, DR.BENCH, as a new benchmark for developing and evaluating cNLP models with clinical diagnostic reasoning ability. The suite includes six tasks from ten publicly available datasets addressing clinical text understanding, medical knowledge reasoning, and diagnosis generation. DR.BENCH is the first clinical suite of tasks designed to be a natural language generation framework to evaluate pre-trained language models. Experiments with state-of-the-art pre-trained generative language models using large general domain models and models that were continually trained on a medical corpus demonstrate opportunities for improvement when evaluated in DR. BENCH. We share DR. BENCH as a publicly available GitLab repository with a systematic approach to load and evaluate models for the cNLP community.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge