Dimitrios Karkalousos
ATOMMIC: An Advanced Toolbox for Multitask Medical Imaging Consistency to facilitate Artificial Intelligence applications from acquisition to analysis in Magnetic Resonance Imaging
Apr 30, 2024Abstract:AI is revolutionizing MRI along the acquisition and processing chain. Advanced AI frameworks have been developed to apply AI in various successive tasks, such as image reconstruction, quantitative parameter map estimation, and image segmentation. Existing frameworks are often designed to perform tasks independently or are focused on specific models or datasets, limiting generalization. We introduce ATOMMIC, an open-source toolbox that streamlines AI applications for accelerated MRI reconstruction and analysis. ATOMMIC implements several tasks using DL networks and enables MultiTask Learning (MTL) to perform related tasks integrated, targeting generalization in the MRI domain. We first review the current state of AI frameworks for MRI through a comprehensive literature search and by parsing 12,479 GitHub repositories. We benchmark 25 DL models on eight publicly available datasets to present distinct applications of ATOMMIC on accelerated MRI reconstruction, image segmentation, quantitative parameter map estimation, and joint accelerated MRI reconstruction and image segmentation utilizing MTL. Our findings demonstrate that ATOMMIC is the only MTL framework with harmonized complex-valued and real-valued data support. Evaluations on single tasks show that physics-based models, which enforce data consistency by leveraging the physical properties of MRI, outperform other models in reconstructing highly accelerated acquisitions. Physics-based models that produce high reconstruction quality can accurately estimate quantitative parameter maps. When high-performing reconstruction models are combined with robust segmentation networks utilizing MTL, performance is improved in both tasks. ATOMMIC facilitates MRI reconstruction and analysis by standardizing workflows, enhancing data interoperability, integrating unique features like MTL, and effectively benchmarking DL models.
State-of-the-Art Machine Learning MRI Reconstruction in 2020: Results of the Second fastMRI Challenge
Dec 28, 2020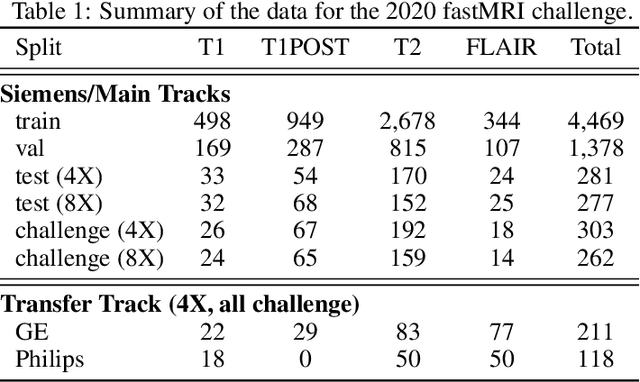
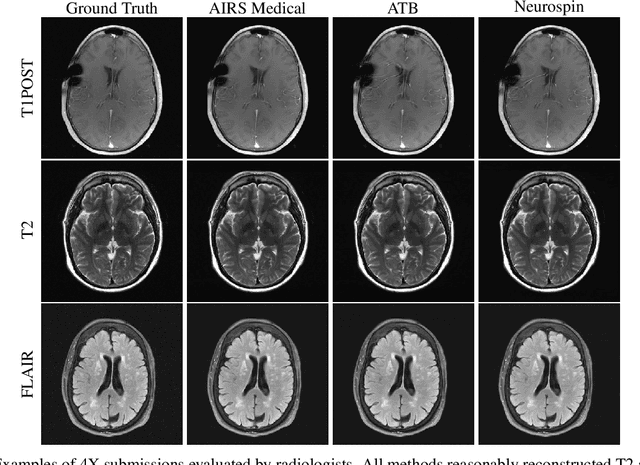
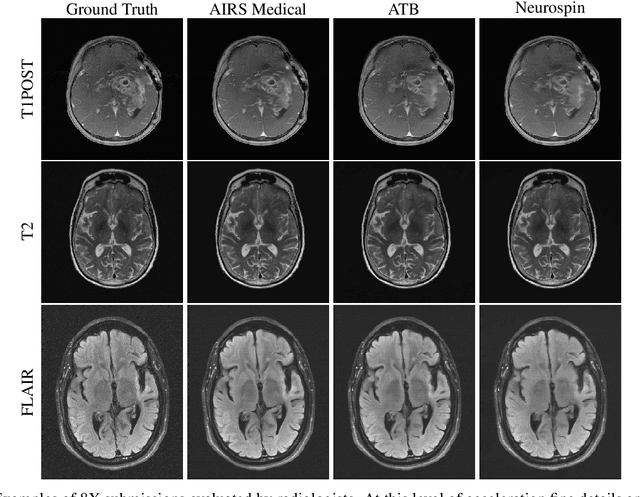
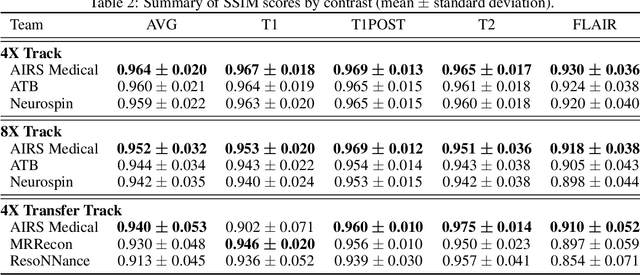
Abstract:Accelerating MRI scans is one of the principal outstanding problems in the MRI research community. Towards this goal, we hosted the second fastMRI competition targeted towards reconstructing MR images with subsampled k-space data. We provided participants with data from 7,299 clinical brain scans (de-identified via a HIPAA-compliant procedure by NYU Langone Health), holding back the fully-sampled data from 894 of these scans for challenge evaluation purposes. In contrast to the 2019 challenge, we focused our radiologist evaluations on pathological assessment in brain images. We also debuted a new Transfer track that required participants to submit models evaluated on MRI scanners from outside the training set. We received 19 submissions from eight different groups. Results showed one team scoring best in both SSIM scores and qualitative radiologist evaluations. We also performed analysis on alternative metrics to mitigate the effects of background noise and collected feedback from the participants to inform future challenges. Lastly, we identify common failure modes across the submissions, highlighting areas of need for future research in the MRI reconstruction community.
Multi-channel MR Reconstruction (MC-MRRec) Challenge -- Comparing Accelerated MR Reconstruction Models and Assessing Their Genereralizability to Datasets Collected with Different Coils
Nov 10, 2020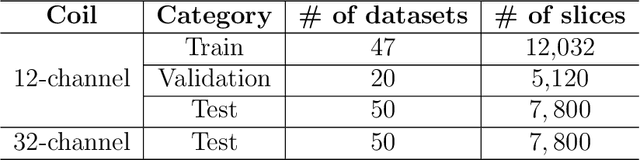
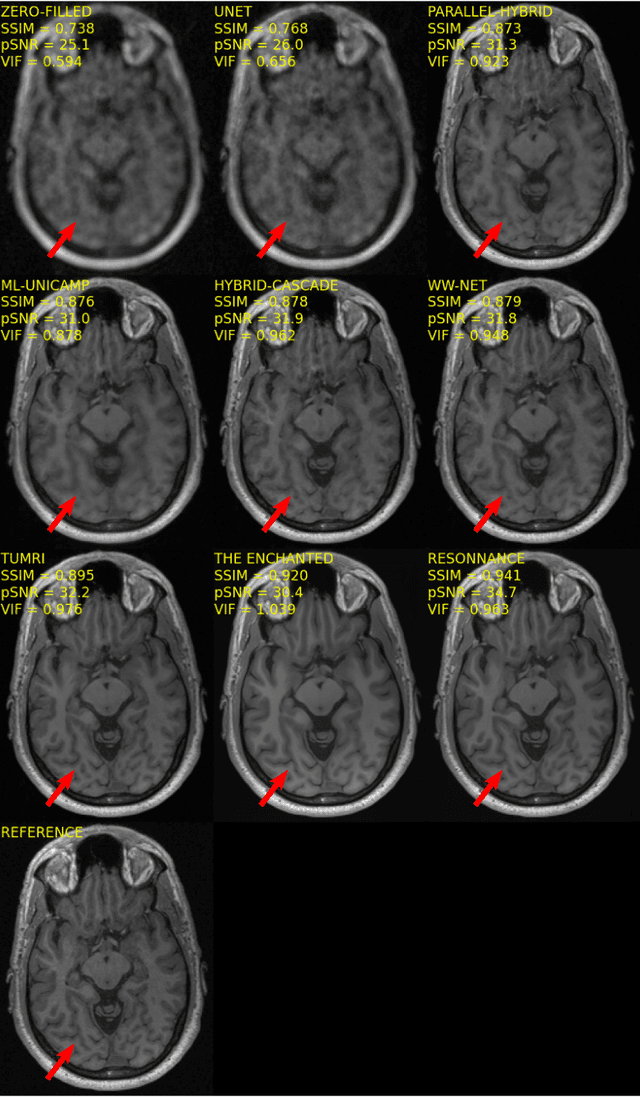
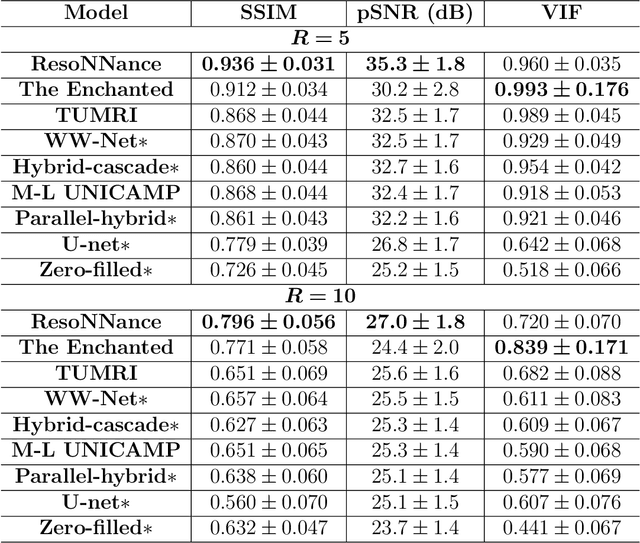
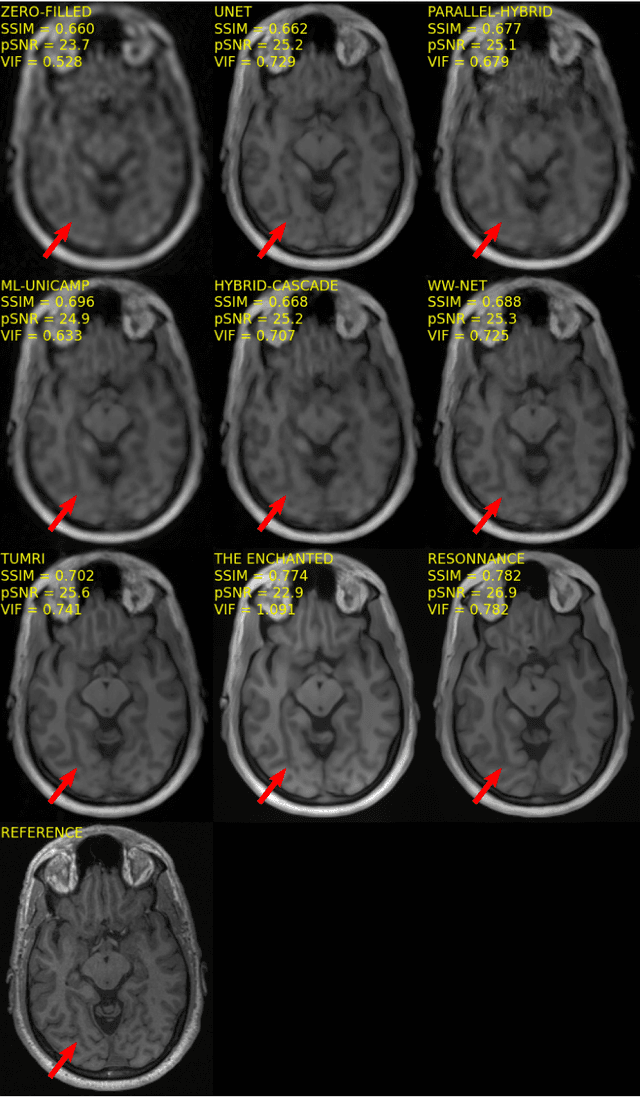
Abstract:The 2020 Multi-channel Magnetic Resonance Reconstruction (MC-MRRec) Challenge had two primary goals: 1) compare different MR image reconstruction models on a large dataset and 2) assess the generalizability of these models to datasets acquired with a different number of receiver coils (i.e., multiple channels). The challenge had two tracks: Track 01 focused on assessing models trained and tested with 12-channel data. Track 02 focused on assessing models trained with 12-channel data and tested on both 12-channel and 32-channel data. While the challenge is ongoing, here we describe the first edition of the challenge and summarise submissions received prior to 5 September 2020. Track 01 had five baseline models and received four independent submissions. Track 02 had two baseline models and received two independent submissions. This manuscript provides relevant comparative information on the current state-of-the-art of MR reconstruction and highlights the challenges of obtaining generalizable models that are required prior to clinical adoption. Both challenge tracks remain open and will provide an objective performance assessment for future submissions. Subsequent editions of the challenge are proposed to investigate new concepts and strategies, such as the integration of potentially available longitudinal information during the MR reconstruction process. An outline of the proposed second edition of the challenge is presented in this manuscript.
i-RIM applied to the fastMRI challenge
Oct 20, 2019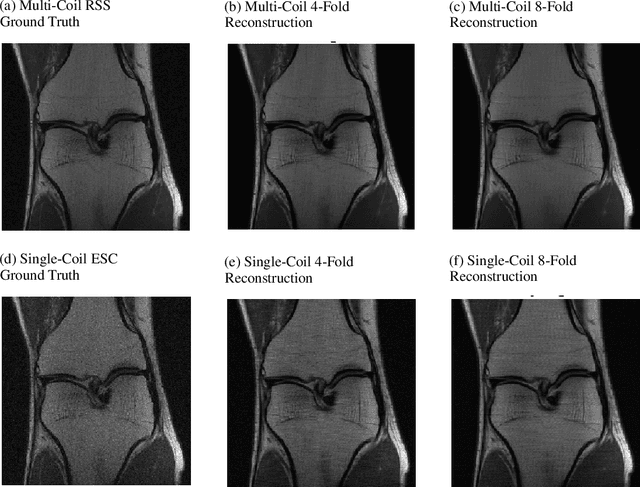
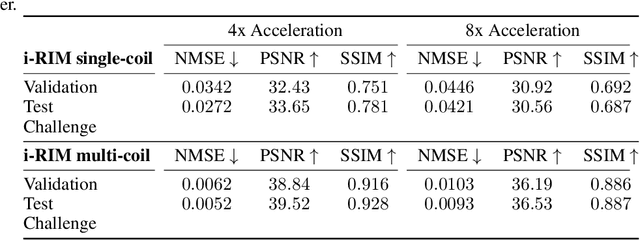
Abstract:We, team AImsterdam, summarize our submission to the fastMRI challenge (Zbontar et al., 2018). Our approach builds on recent advances in invertible learning to infer models as presented in Putzky and Welling (2019). Both, our single-coil and our multi-coil model share the same basic architecture.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge