Geunu Jeong
All-in-One Deep Learning Framework for MR Image Reconstruction
May 06, 2024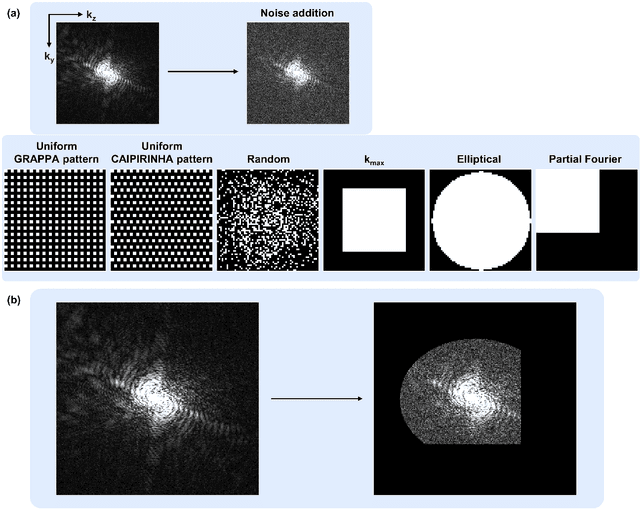
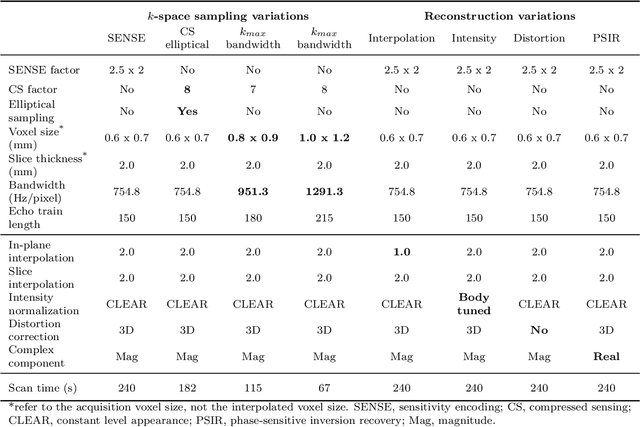
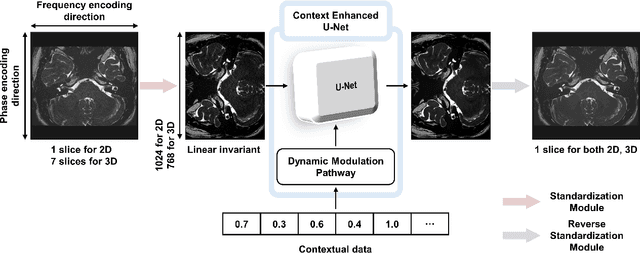
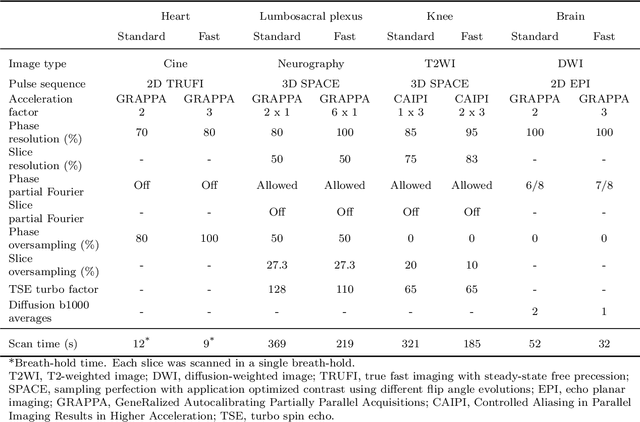
Abstract:We introduce a novel, all-in-one deep learning framework for MR image reconstruction, enabling a single model to enhance image quality across multiple aspects of k-space sampling and to be effective across a wide range of clinical and technical scenarios. This DICOM-based algorithm serves as the core of SwiftMR (AIRS Medical, Seoul, Korea), which is FDA-cleared, CE-certified, and commercially available. We first detail the comprehensive development process of the model, including data collection, training pair preparation, model architecture design, and DICOM inference. We then assess the model's capability to enhance image quality in a multi-dimensional manner, specifically across various aspects of k-space sampling. Subsequently, we evaluate several features of the multi-dimensional enhancement: the accuracy of tunable denoising, the effectiveness of super-resolution in each encoding direction, and the reduction of artifacts that become more prominent at lower spatial resolutions. Additionally, we assess its compatibility with various scan parameter sets and its generalizability across scanner vendors not seen during training. Finally, we present specific cases demonstrating the model's utility in reducing scan time across anatomical regions in conjunction with protocol optimization. The proposed model is compatible with a broad spectrum of scenarios, including various vendors, pulse sequences, scan parameters, and anatomical regions. Its DICOM-based operation particularly enhances its applicability for real-world applications. Given its demonstrated effectiveness and versatility, we expect its use to expand in the field of clinical MRI.
State-of-the-Art Machine Learning MRI Reconstruction in 2020: Results of the Second fastMRI Challenge
Dec 28, 2020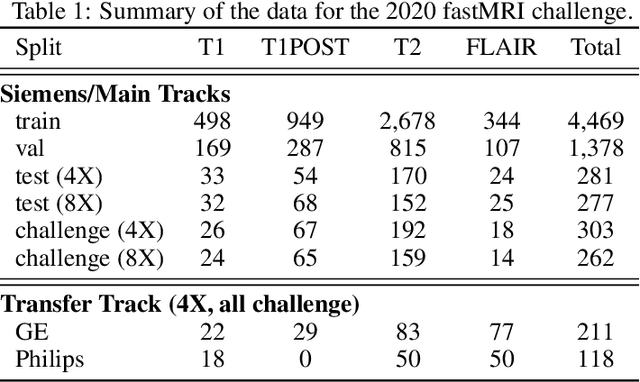
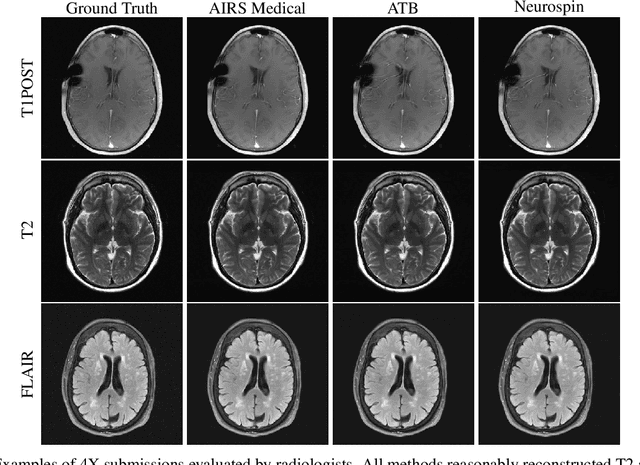
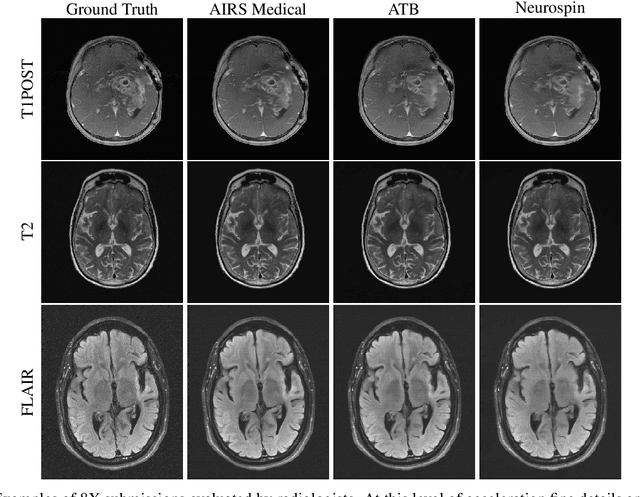
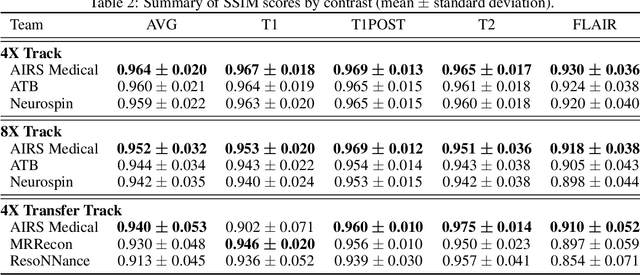
Abstract:Accelerating MRI scans is one of the principal outstanding problems in the MRI research community. Towards this goal, we hosted the second fastMRI competition targeted towards reconstructing MR images with subsampled k-space data. We provided participants with data from 7,299 clinical brain scans (de-identified via a HIPAA-compliant procedure by NYU Langone Health), holding back the fully-sampled data from 894 of these scans for challenge evaluation purposes. In contrast to the 2019 challenge, we focused our radiologist evaluations on pathological assessment in brain images. We also debuted a new Transfer track that required participants to submit models evaluated on MRI scanners from outside the training set. We received 19 submissions from eight different groups. Results showed one team scoring best in both SSIM scores and qualitative radiologist evaluations. We also performed analysis on alternative metrics to mitigate the effects of background noise and collected feedback from the participants to inform future challenges. Lastly, we identify common failure modes across the submissions, highlighting areas of need for future research in the MRI reconstruction community.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge