Matthan W. A. Caan
ATOMMIC: An Advanced Toolbox for Multitask Medical Imaging Consistency to facilitate Artificial Intelligence applications from acquisition to analysis in Magnetic Resonance Imaging
Apr 30, 2024Abstract:AI is revolutionizing MRI along the acquisition and processing chain. Advanced AI frameworks have been developed to apply AI in various successive tasks, such as image reconstruction, quantitative parameter map estimation, and image segmentation. Existing frameworks are often designed to perform tasks independently or are focused on specific models or datasets, limiting generalization. We introduce ATOMMIC, an open-source toolbox that streamlines AI applications for accelerated MRI reconstruction and analysis. ATOMMIC implements several tasks using DL networks and enables MultiTask Learning (MTL) to perform related tasks integrated, targeting generalization in the MRI domain. We first review the current state of AI frameworks for MRI through a comprehensive literature search and by parsing 12,479 GitHub repositories. We benchmark 25 DL models on eight publicly available datasets to present distinct applications of ATOMMIC on accelerated MRI reconstruction, image segmentation, quantitative parameter map estimation, and joint accelerated MRI reconstruction and image segmentation utilizing MTL. Our findings demonstrate that ATOMMIC is the only MTL framework with harmonized complex-valued and real-valued data support. Evaluations on single tasks show that physics-based models, which enforce data consistency by leveraging the physical properties of MRI, outperform other models in reconstructing highly accelerated acquisitions. Physics-based models that produce high reconstruction quality can accurately estimate quantitative parameter maps. When high-performing reconstruction models are combined with robust segmentation networks utilizing MTL, performance is improved in both tasks. ATOMMIC facilitates MRI reconstruction and analysis by standardizing workflows, enhancing data interoperability, integrating unique features like MTL, and effectively benchmarking DL models.
Deep Learning-based Intraoperative MRI Reconstruction
Jan 23, 2024Abstract:Purpose: To evaluate the quality of deep learning reconstruction for prospectively accelerated intraoperative magnetic resonance imaging (iMRI) during resective brain tumor surgery. Materials and Methods: Accelerated iMRI was performed during brain surgery using dual surface coils positioned around the area of resection. A deep learning (DL) model was trained on the fastMRI neuro dataset to mimic the data from the iMRI protocol. Evaluation was performed on imaging material from 40 patients imaged between 01.11.2021 - 01.06.2023 that underwent iMRI during tumor resection surgery. A comparative analysis was conducted between the conventional compressed sense (CS) method and the trained DL reconstruction method. Blinded evaluation of multiple image quality metrics was performed by two working neuro-radiologists and a working neurosurgeon on a 1 to 5 Likert scale (1=non diagnostic, 2=poor, 3=acceptable, 4=good, 5=excellent), and the favored reconstruction variant. Results: The DL reconstruction was strongly favored or favored over the CS reconstruction for 33/40, 39/40, and 8/40 of cases for reader 1, 2, and 3, respectively. Two of three readers consistently assigned higher ratings for the DL reconstructions, and the DL reconstructions had a higher score than their respective CS counterparts for 72%, 72%, and 14% of the cases for reader 1, 2, and 3, respectively. Still, the DL reconstructions exhibited shortcomings such as a striping artifact and reduced signal. Conclusion: DL shows promise to allow for high-quality reconstructions of intraoperative MRI with equal to or improved perceived spatial resolution, signal-to-noise ratio, diagnostic confidence, diagnostic conspicuity, and spatial resolution compared to compressed sense.
A Densely Interconnected Network for Deep Learning Accelerated MRI
Jul 05, 2022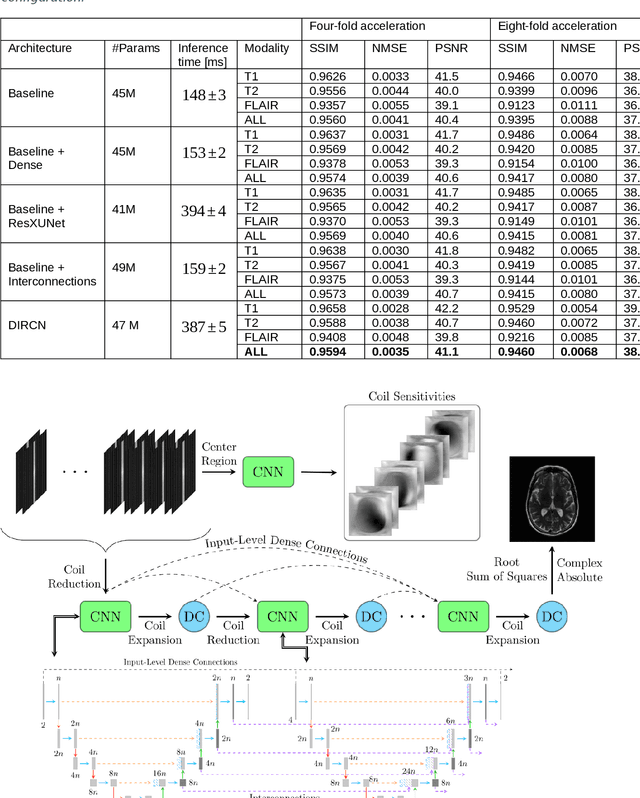
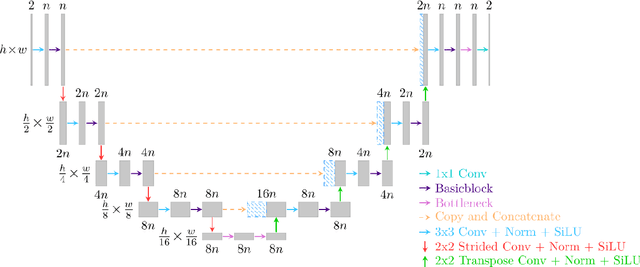
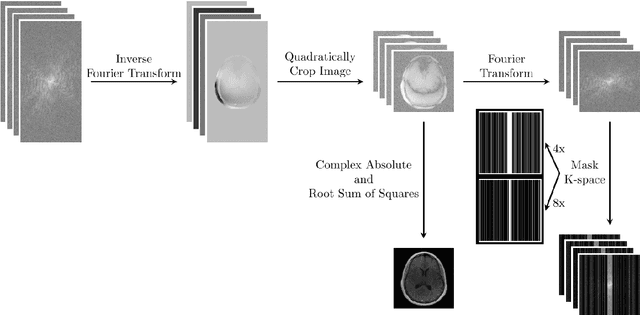
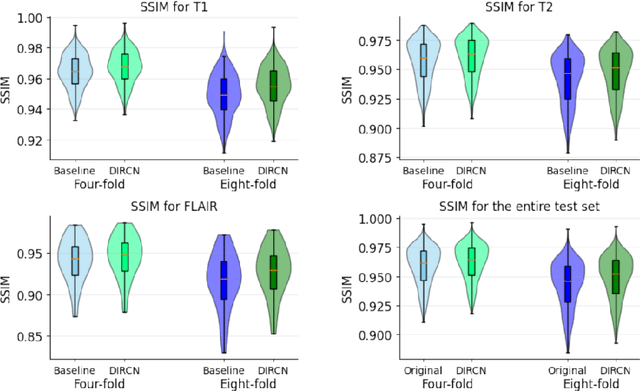
Abstract:Objective: To improve accelerated MRI reconstruction through a densely connected cascading deep learning reconstruction framework. Materials and Methods: A cascading deep learning reconstruction framework (baseline model) was modified by applying three architectural modifications: Input-level dense connections between cascade inputs and outputs, an improved deep learning sub-network, and long-range skip-connections between subsequent deep learning networks. An ablation study was performed, where five model configurations were trained on the NYU fastMRI neuro dataset with an end-to-end scheme conjunct on four- and eight-fold acceleration. The trained models were evaluated by comparing their respective structural similarity index measure (SSIM), normalized mean square error (NMSE) and peak signal to noise ratio (PSNR). Results: The proposed densely interconnected residual cascading network (DIRCN), utilizing all three suggested modifications, achieved a SSIM improvement of 8% and 11% for four- and eight-fold acceleration, respectively. For eight-fold acceleration, the model achieved a 23% decrease in the NMSE when compared to the baseline model. In an ablation study, the individual architectural modifications all contributed to this improvement, by reducing the SSIM and NMSE with approximately 3% and 5% for four-fold acceleration, respectively. Conclusion: The proposed architectural modifications allow for simple adjustments on an already existing cascading framework to further improve the resulting reconstructions.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge