Damini Dey
World of Forms: Deformable Geometric Templates for One-Shot Surface Meshing in Coronary CT Angiography
Sep 18, 2024Abstract:Deep learning-based medical image segmentation and surface mesh generation typically involve a sequential pipeline from image to segmentation to meshes, often requiring large training datasets while making limited use of prior geometric knowledge. This may lead to topological inconsistencies and suboptimal performance in low-data regimes. To address these challenges, we propose a data-efficient deep learning method for direct 3D anatomical object surface meshing using geometric priors. Our approach employs a multi-resolution graph neural network that operates on a prior geometric template which is deformed to fit object boundaries of interest. We show how different templates may be used for the different surface meshing targets, and introduce a novel masked autoencoder pretraining strategy for 3D spherical data. The proposed method outperforms nnUNet in a one-shot setting for segmentation of the pericardium, left ventricle (LV) cavity and the LV myocardium. Similarly, the method outperforms other lumen segmentation operating on multi-planar reformatted images. Results further indicate that mesh quality is on par with or improves upon marching cubes post-processing of voxel mask predictions, while remaining flexible in the choice of mesh triangulation prior, thus paving the way for more accurate and topologically consistent 3D medical object surface meshing.
Rapid quantification of COVID-19 pneumonia burden from computed tomography with convolutional LSTM networks
Mar 31, 2021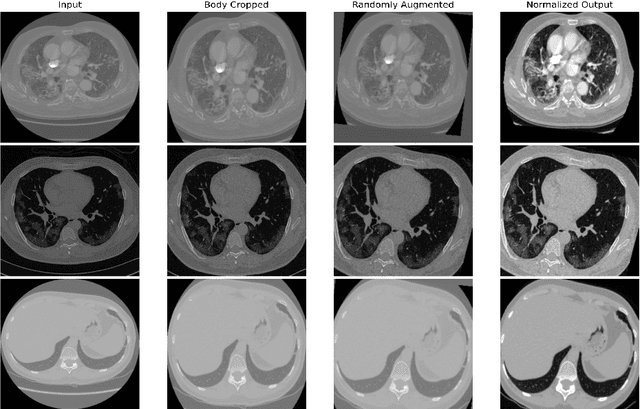
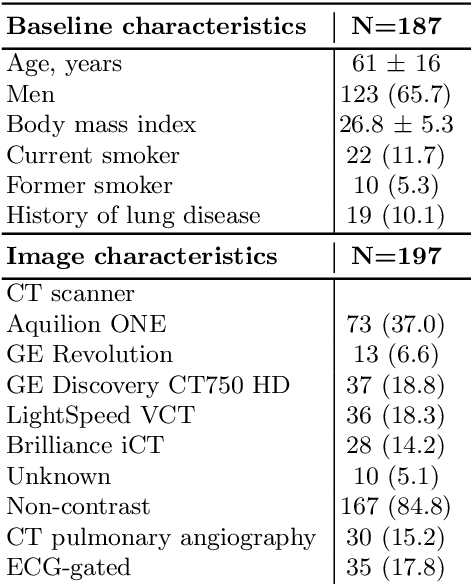
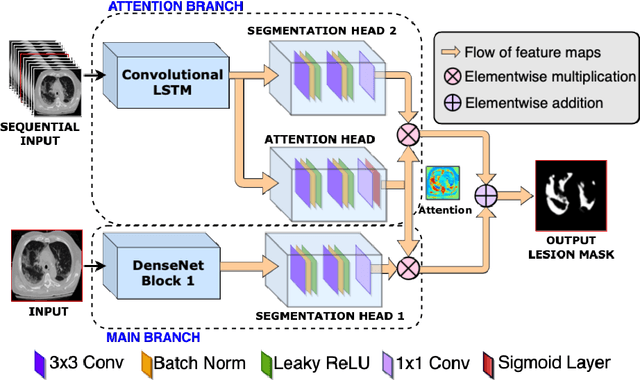
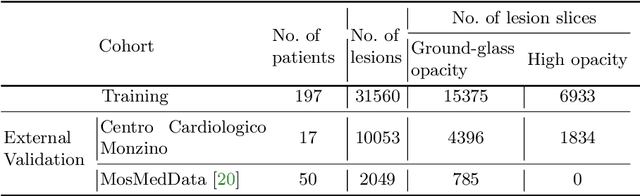
Abstract:Quantitative lung measures derived from computed tomography (CT) have been demonstrated to improve prognostication in coronavirus disease (COVID-19) patients, but are not part of the clinical routine since required manual segmentation of lung lesions is prohibitively time-consuming. We propose a new fully automated deep learning framework for rapid quantification and differentiation between lung lesions in COVID-19 pneumonia from both contrast and non-contrast CT images using convolutional Long Short-Term Memory (ConvLSTM) networks. Utilizing the expert annotations, model training was performed 5 times with separate hold-out sets using 5-fold cross-validation to segment ground-glass opacity and high opacity (including consolidation and pleural effusion). The performance of the method was evaluated on CT data sets from 197 patients with positive reverse transcription polymerase chain reaction test result for SARS-CoV-2. Strong agreement between expert manual and automatic segmentation was obtained for lung lesions with a Dice score coefficient of 0.876 $\pm$ 0.005; excellent correlations of 0.978 and 0.981 for ground-glass opacity and high opacity volumes. In the external validation set of 67 patients, there was dice score coefficient of 0.767 $\pm$ 0.009 as well as excellent correlations of 0.989 and 0.996 for ground-glass opacity and high opacity volumes. Computations for a CT scan comprising 120 slices were performed under 2 seconds on a personal computer equipped with NVIDIA Titan RTX graphics processing unit. Therefore, our deep learning-based method allows rapid fully-automated quantitative measurement of pneumonia burden from CT and may generate results with an accuracy similar to the expert readers.
Calcium Removal From Cardiac CT Images Using Deep Convolutional Neural Network
Feb 20, 2018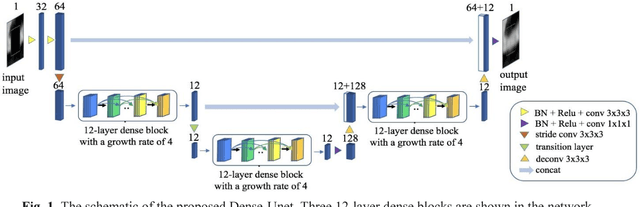
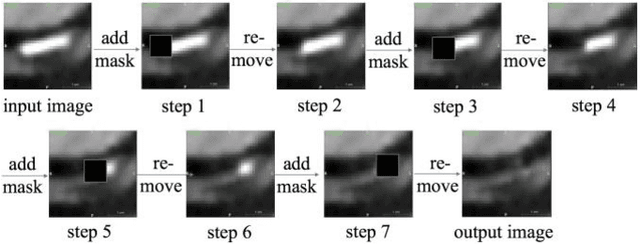
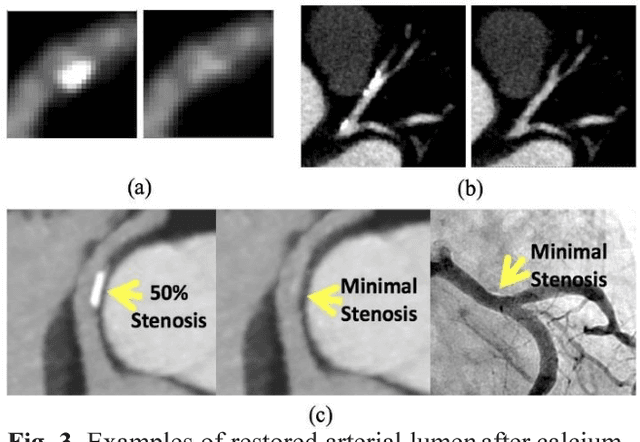

Abstract:Coronary calcium causes beam hardening and blooming artifacts on cardiac computed tomography angiography (CTA) images, which lead to overestimation of lumen stenosis and reduction of diagnostic specificity. To properly remove coronary calcification and restore arterial lumen precisely, we propose a machine learning-based method with a multi-step inpainting process. We developed a new network configuration, Dense-Unet, to achieve optimal performance with low computational cost. Results after the calcium removal process were validated by comparing with gold-standard X-ray angiography. Our results demonstrated that removing coronary calcification from images with the proposed approach was feasible, and may potentially improve the diagnostic accuracy of CTA.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge