Sang-Eun Lee
Spatiotemporal Multi-Camera Calibration using Freely Moving People
Feb 18, 2025



Abstract:We propose a novel method for spatiotemporal multi-camera calibration using freely moving people in multiview videos. Since calibrating multiple cameras and finding matches across their views are inherently interdependent, performing both in a unified framework poses a significant challenge. We address these issues as a single registration problem of matching two sets of 3D points, leveraging human motion in dynamic multi-person scenes. To this end, we utilize 3D human poses obtained from an off-the-shelf monocular 3D human pose estimator and transform them into 3D points on a unit sphere, to solve the rotation, time offset, and the association alternatingly. We employ a probabilistic approach that can jointly solve both problems of aligning spatiotemporal data and establishing correspondences through soft assignment between two views. The translation is determined by applying coplanarity constraints. The pairwise registration results are integrated into a multiview setup, and then a nonlinear optimization method is used to improve the accuracy of the camera poses, temporal offsets, and multi-person associations. Extensive experiments on synthetic and real data demonstrate the effectiveness and flexibility of the proposed method as a practical marker-free calibration tool.
CycleGAN with a Blur Kernel for Deconvolution Microscopy: Optimal Transport Geometry
Aug 26, 2019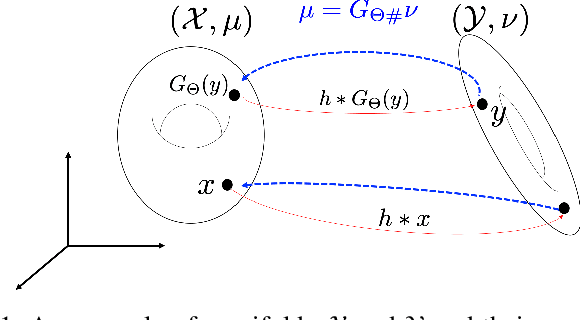
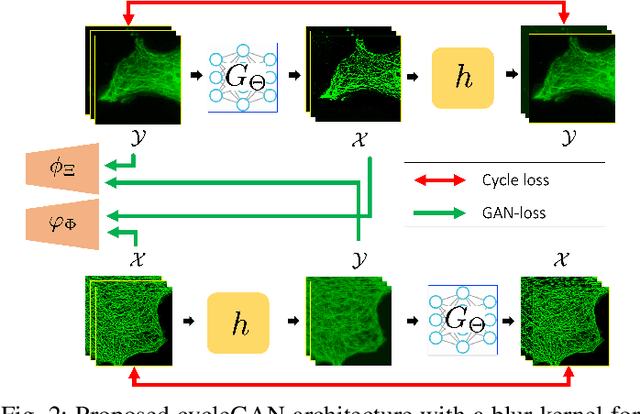
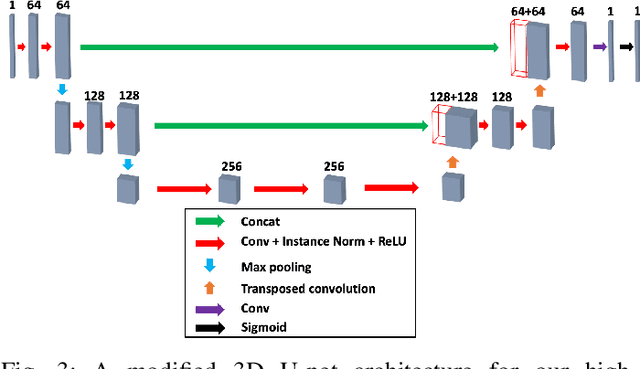
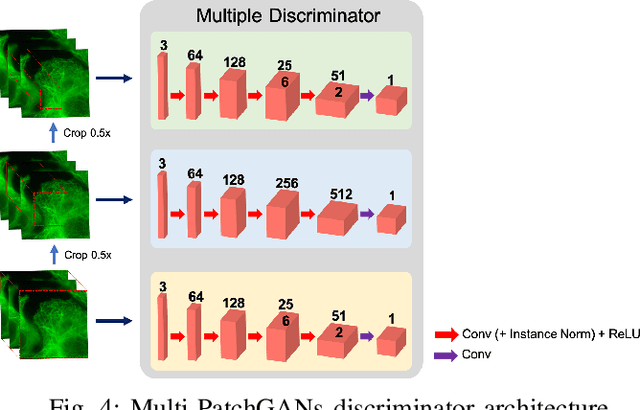
Abstract:Deconvolution microscopy has been extensively used to improve the resolution of the widefield fluorescent microscopy. However, classical deconvolution approaches require the measurement or estimation of the point spread function (PSF), and are usually computationally expensive. Recently, convolutional neural network (CNN) approaches have been extensively studied as fast and high performance alternatives. Unfortunately, the CNN approaches usually require matched high resolution images for supervised training. In this paper, we present a novel unsupervised cycle-consistent generative adversarial network (cycleGAN) with a linear blur kernel, which can be used for both blind- and non-blind image deconvolution. In contrast to the conventional cycleGAN approaches that require two generators, the proposed cycleGAN approach needs only a single generator, which significantly improves the robustness of network training. We show that the proposed architecture is indeed a dual formulation of an optimal transport problem that uses a special form of penalized least squares as transport cost. Experimental results using simulated and real experimental data confirm the efficacy of the algorithm.
Blind Deconvolution Microscopy Using Cycle Consistent CNN with Explicit PSF Layer
Apr 05, 2019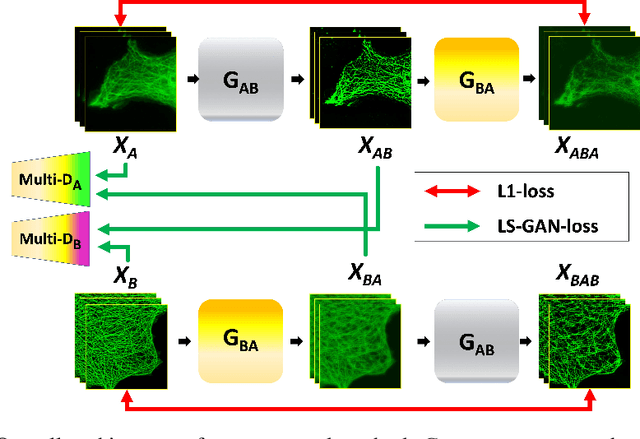
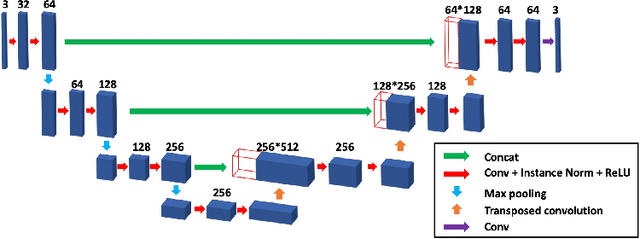
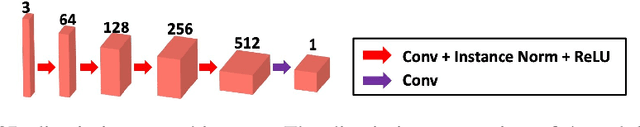
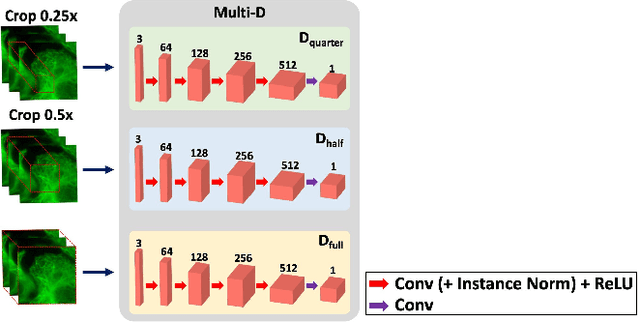
Abstract:Deconvolution microscopy has been extensively used to improve the resolution of the widefield fluorescent microscopy. Conventional approaches, which usually require the point spread function (PSF) measurement or blind estimation, are however computationally expensive. Recently, CNN based approaches have been explored as a fast and high performance alternative. In this paper, we present a novel unsupervised deep neural network for blind deconvolution based on cycle consistency and PSF modeling layers. In contrast to the recent CNN approaches for similar problem, the explicit PSF modeling layers improve the robustness of the algorithm. Experimental results confirm the efficacy of the algorithm.
Calcium Removal From Cardiac CT Images Using Deep Convolutional Neural Network
Feb 20, 2018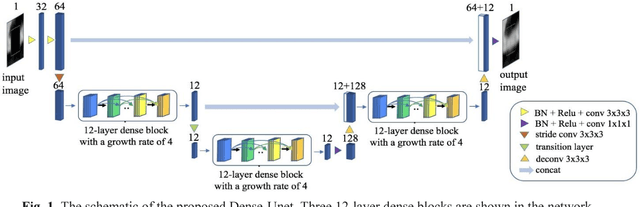
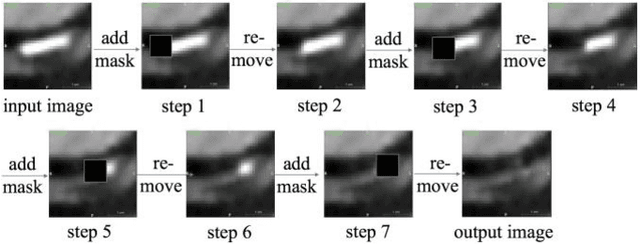
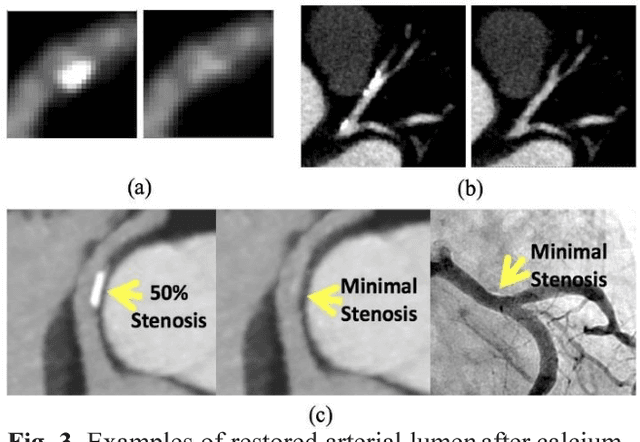

Abstract:Coronary calcium causes beam hardening and blooming artifacts on cardiac computed tomography angiography (CTA) images, which lead to overestimation of lumen stenosis and reduction of diagnostic specificity. To properly remove coronary calcification and restore arterial lumen precisely, we propose a machine learning-based method with a multi-step inpainting process. We developed a new network configuration, Dense-Unet, to achieve optimal performance with low computational cost. Results after the calcium removal process were validated by comparing with gold-standard X-ray angiography. Our results demonstrated that removing coronary calcification from images with the proposed approach was feasible, and may potentially improve the diagnostic accuracy of CTA.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge