Ioannis Lagogiannis
World of Forms: Deformable Geometric Templates for One-Shot Surface Meshing in Coronary CT Angiography
Sep 18, 2024Abstract:Deep learning-based medical image segmentation and surface mesh generation typically involve a sequential pipeline from image to segmentation to meshes, often requiring large training datasets while making limited use of prior geometric knowledge. This may lead to topological inconsistencies and suboptimal performance in low-data regimes. To address these challenges, we propose a data-efficient deep learning method for direct 3D anatomical object surface meshing using geometric priors. Our approach employs a multi-resolution graph neural network that operates on a prior geometric template which is deformed to fit object boundaries of interest. We show how different templates may be used for the different surface meshing targets, and introduce a novel masked autoencoder pretraining strategy for 3D spherical data. The proposed method outperforms nnUNet in a one-shot setting for segmentation of the pericardium, left ventricle (LV) cavity and the LV myocardium. Similarly, the method outperforms other lumen segmentation operating on multi-planar reformatted images. Results further indicate that mesh quality is on par with or improves upon marching cubes post-processing of voxel mask predictions, while remaining flexible in the choice of mesh triangulation prior, thus paving the way for more accurate and topologically consistent 3D medical object surface meshing.
Unsupervised Pathology Detection: A Deep Dive Into the State of the Art
Mar 01, 2023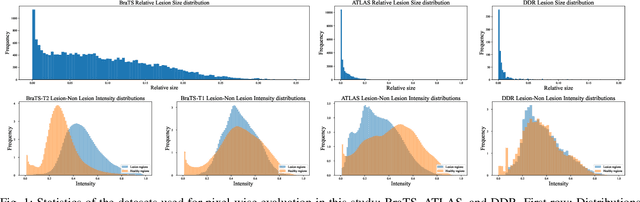
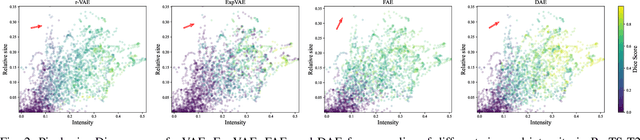

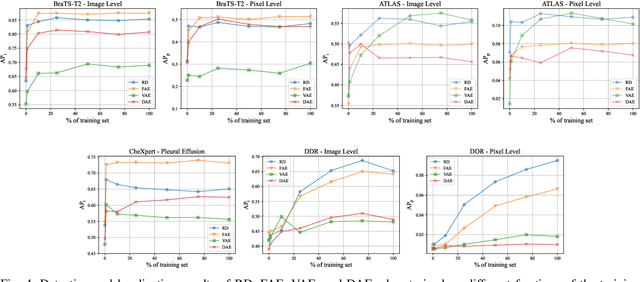
Abstract:Deep unsupervised approaches are gathering increased attention for applications such as pathology detection and segmentation in medical images since they promise to alleviate the need for large labeled datasets and are more generalizable than their supervised counterparts in detecting any kind of rare pathology. As the Unsupervised Anomaly Detection (UAD) literature continuously grows and new paradigms emerge, it is vital to continuously evaluate and benchmark new methods in a common framework, in order to reassess the state-of-the-art (SOTA) and identify promising research directions. To this end, we evaluate a diverse selection of cutting-edge UAD methods on multiple medical datasets, comparing them against the established SOTA in UAD for brain MRI. Our experiments demonstrate that newly developed feature-modeling methods from the industrial and medical literature achieve increased performance compared to previous work and set the new SOTA in a variety of modalities and datasets. Additionally, we show that such methods are capable of benefiting from recently developed self-supervised pre-training algorithms, further increasing their performance. Finally, we perform a series of experiments in order to gain further insights into some unique characteristics of selected models and datasets. Our code can be found under https://github.com/iolag/UPD_study/.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge