Alexander Radbruch
Efficient MedSAMs: Segment Anything in Medical Images on Laptop
Dec 20, 2024


Abstract:Promptable segmentation foundation models have emerged as a transformative approach to addressing the diverse needs in medical images, but most existing models require expensive computing, posing a big barrier to their adoption in clinical practice. In this work, we organized the first international competition dedicated to promptable medical image segmentation, featuring a large-scale dataset spanning nine common imaging modalities from over 20 different institutions. The top teams developed lightweight segmentation foundation models and implemented an efficient inference pipeline that substantially reduced computational requirements while maintaining state-of-the-art segmentation accuracy. Moreover, the post-challenge phase advanced the algorithms through the design of performance booster and reproducibility tasks, resulting in improved algorithms and validated reproducibility of the winning solution. Furthermore, the best-performing algorithms have been incorporated into the open-source software with a user-friendly interface to facilitate clinical adoption. The data and code are publicly available to foster the further development of medical image segmentation foundation models and pave the way for impactful real-world applications.
Pre-examinations Improve Automated Metastases Detection on Cranial MRI
Mar 13, 2024Abstract:Materials and methods: First, a dual-time approach was assessed, for which the CNN was provided sequences of the MRI that initially depicted new MM (diagnosis MRI) as well as of a prediagnosis MRI: inclusion of only contrast-enhanced T1-weighted images (CNNdual_ce) was compared with inclusion of also the native T1-weighted images, T2-weighted images, and FLAIR sequences of both time points (CNNdual_all).Second, results were compared with the corresponding single time approaches, in which the CNN was provided exclusively the respective sequences of the diagnosis MRI.Casewise diagnostic performance parameters were calculated from 5-fold cross-validation. Results: In total, 94 cases with 494 MMs were included. Overall, the highest diagnostic performance was achieved by inclusion of only the contrast-enhanced T1-weighted images of the diagnosis and of a prediagnosis MRI (CNNdual_ce, sensitivity = 73%, PPV = 25%, F1-score = 36%). Using exclusively contrast-enhanced T1-weighted images as input resulted in significantly less false-positives (FPs) compared with inclusion of further sequences beyond contrast-enhanced T1-weighted images (FPs = 5/7 for CNNdual_ce/CNNdual_all, P < 1e-5). Comparison of contrast-enhanced dual and mono time approaches revealed that exclusion of prediagnosis MRI significantly increased FPs (FPs = 5/10 for CNNdual_ce/CNNce, P < 1e-9).Approaches with only native sequences were clearly inferior to CNNs that were provided contrast-enhanced sequences. Conclusions: Automated MM detection on contrast-enhanced T1-weighted images performed with high sensitivity. Frequent FPs due to artifacts and vessels were significantly reduced by additional inclusion of prediagnosis MRI, but not by inclusion of further sequences beyond contrast-enhanced T1-weighted images. Future studies might investigate different change detection architectures for computer-aided detection.
Gadolinium dose reduction for brain MRI using conditional deep learning
Mar 06, 2024



Abstract:Recently, deep learning (DL)-based methods have been proposed for the computational reduction of gadolinium-based contrast agents (GBCAs) to mitigate adverse side effects while preserving diagnostic value. Currently, the two main challenges for these approaches are the accurate prediction of contrast enhancement and the synthesis of realistic images. In this work, we address both challenges by utilizing the contrast signal encoded in the subtraction images of pre-contrast and post-contrast image pairs. To avoid the synthesis of any noise or artifacts and solely focus on contrast signal extraction and enhancement from low-dose subtraction images, we train our DL model using noise-free standard-dose subtraction images as targets. As a result, our model predicts the contrast enhancement signal only; thereby enabling synthesization of images beyond the standard dose. Furthermore, we adapt the embedding idea of recent diffusion-based models to condition our model on physical parameters affecting the contrast enhancement behavior. We demonstrate the effectiveness of our approach on synthetic and real datasets using various scanners, field strengths, and contrast agents.
Faithful Synthesis of Low-dose Contrast-enhanced Brain MRI Scans using Noise-preserving Conditional GANs
Jun 26, 2023Abstract:Today Gadolinium-based contrast agents (GBCA) are indispensable in Magnetic Resonance Imaging (MRI) for diagnosing various diseases. However, GBCAs are expensive and may accumulate in patients with potential side effects, thus dose-reduction is recommended. Still, it is unclear to which extent the GBCA dose can be reduced while preserving the diagnostic value -- especially in pathological regions. To address this issue, we collected brain MRI scans at numerous non-standard GBCA dosages and developed a conditional GAN model for synthesizing corresponding images at fractional dose levels. Along with the adversarial loss, we advocate a novel content loss function based on the Wasserstein distance of locally paired patch statistics for the faithful preservation of noise. Our numerical experiments show that conditional GANs are suitable for generating images at different GBCA dose levels and can be used to augment datasets for virtual contrast models. Moreover, our model can be transferred to openly available datasets such as BraTS, where non-standard GBCA dosage images do not exist.
Revealing Hidden Potentials of the q-Space Signal in Breast Cancer
May 22, 2017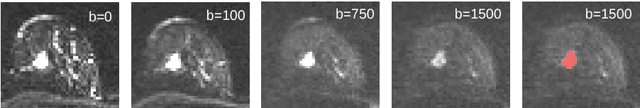

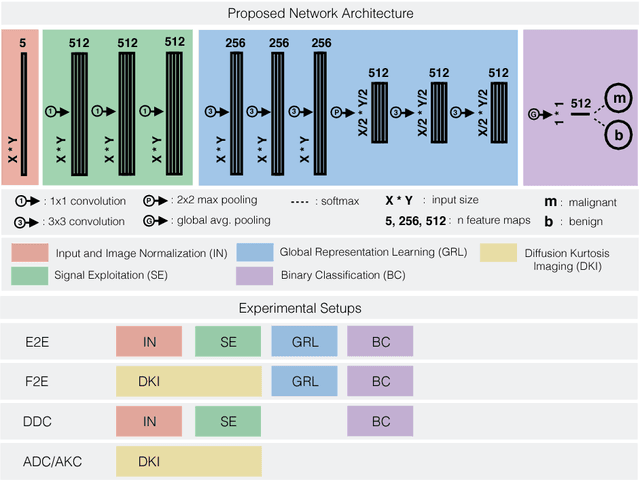
Abstract:Mammography screening for early detection of breast lesions currently suffers from high amounts of false positive findings, which result in unnecessary invasive biopsies. Diffusion-weighted MR images (DWI) can help to reduce many of these false-positive findings prior to biopsy. Current approaches estimate tissue properties by means of quantitative parameters taken from generative, biophysical models fit to the q-space encoded signal under certain assumptions regarding noise and spatial homogeneity. This process is prone to fitting instability and partial information loss due to model simplicity. We reveal unexplored potentials of the signal by integrating all data processing components into a convolutional neural network (CNN) architecture that is designed to propagate clinical target information down to the raw input images. This approach enables simultaneous and target-specific optimization of image normalization, signal exploitation, global representation learning and classification. Using a multicentric data set of 222 patients, we demonstrate that our approach significantly improves clinical decision making with respect to the current state of the art.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge