Sebastian Bickelhaupt
Adapted Foundation Models for Breast MRI Triaging in Contrast-Enhanced and Non-Contrast Enhanced Protocols
Nov 08, 2025Abstract:Background: Magnetic resonance imaging (MRI) has high sensitivity for breast cancer detection, but interpretation is time-consuming. Artificial intelligence may aid in pre-screening. Purpose: To evaluate the DINOv2-based Medical Slice Transformer (MST) for ruling out significant findings (Breast Imaging Reporting and Data System [BI-RADS] >=4) in contrast-enhanced and non-contrast-enhanced abbreviated breast MRI. Materials and Methods: This institutional review board approved retrospective study included 1,847 single-breast MRI examinations (377 BI-RADS >=4) from an in-house dataset and 924 from an external validation dataset (Duke). Four abbreviated protocols were tested: T1-weighted early subtraction (T1sub), diffusion-weighted imaging with b=1500 s/mm2 (DWI1500), DWI1500+T2-weighted (T2w), and T1sub+T2w. Performance was assessed at 90%, 95%, and 97.5% sensitivity using five-fold cross-validation and area under the receiver operating characteristic curve (AUC) analysis. AUC differences were compared with the DeLong test. False negatives were characterized, and attention maps of true positives were rated in the external dataset. Results: A total of 1,448 female patients (mean age, 49 +/- 12 years) were included. T1sub+T2w achieved an AUC of 0.77 +/- 0.04; DWI1500+T2w, 0.74 +/- 0.04 (p=0.15). At 97.5% sensitivity, T1sub+T2w had the highest specificity (19% +/- 7%), followed by DWI1500+T2w (17% +/- 11%). Missed lesions had a mean diameter <10 mm at 95% and 97.5% thresholds for both T1sub and DWI1500, predominantly non-mass enhancements. External validation yielded an AUC of 0.77, with 88% of attention maps rated good or moderate. Conclusion: At 97.5% sensitivity, the MST framework correctly triaged cases without BI-RADS >=4, achieving 19% specificity for contrast-enhanced and 17% for non-contrast-enhanced MRI. Further research is warranted before clinical implementation.
Towards Super-Resolution CEST MRI for Visualization of Small Structures
Dec 03, 2021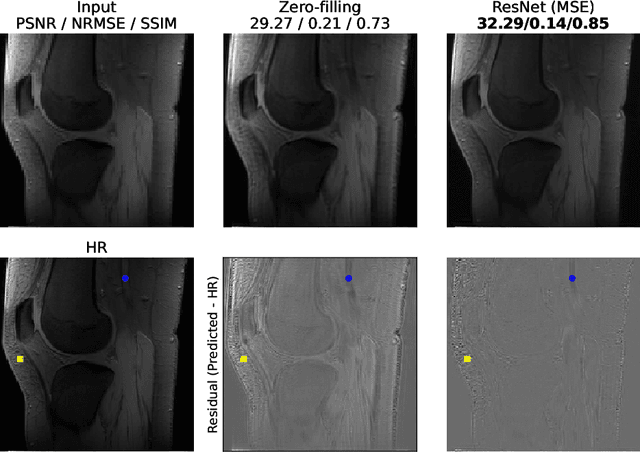
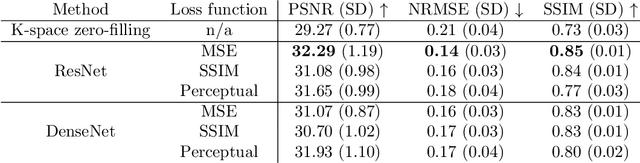
Abstract:The onset of rheumatic diseases such as rheumatoid arthritis is typically subclinical, which results in challenging early detection of the disease. However, characteristic changes in the anatomy can be detected using imaging techniques such as MRI or CT. Modern imaging techniques such as chemical exchange saturation transfer (CEST) MRI drive the hope to improve early detection even further through the imaging of metabolites in the body. To image small structures in the joints of patients, typically one of the first regions where changes due to the disease occur, a high resolution for the CEST MR imaging is necessary. Currently, however, CEST MR suffers from an inherently low resolution due to the underlying physical constraints of the acquisition. In this work we compared established up-sampling techniques to neural network-based super-resolution approaches. We could show, that neural networks are able to learn the mapping from low-resolution to high-resolution unsaturated CEST images considerably better than present methods. On the test set a PSNR of 32.29dB (+10%), a NRMSE of 0.14 (+28%), and a SSIM of 0.85 (+15%) could be achieved using a ResNet neural network, improving the baseline considerably. This work paves the way for the prospective investigation of neural networks for super-resolution CEST MRI and, followingly, might lead to a earlier detection of the onset of rheumatic diseases.
Retina U-Net: Embarrassingly Simple Exploitation of Segmentation Supervision for Medical Object Detection
Nov 21, 2018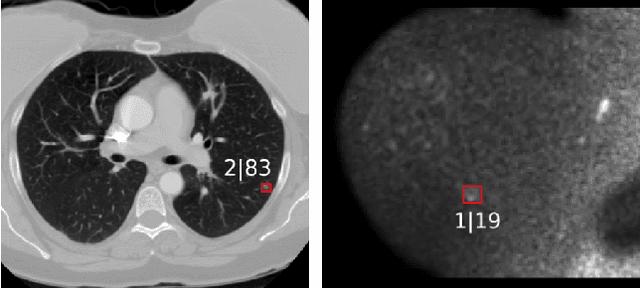
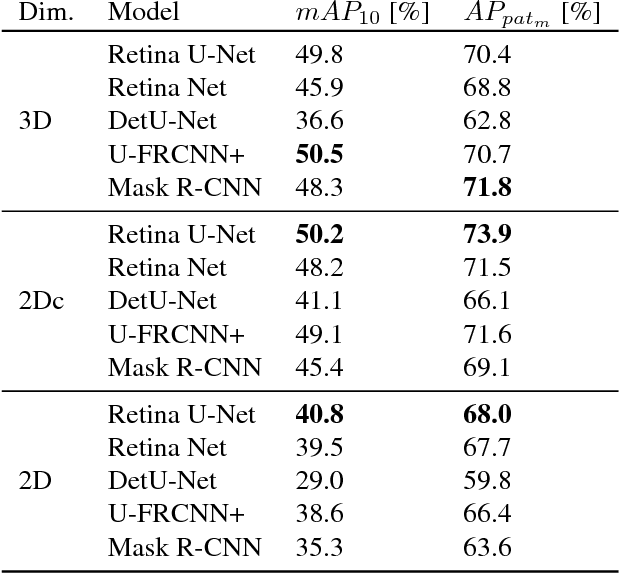
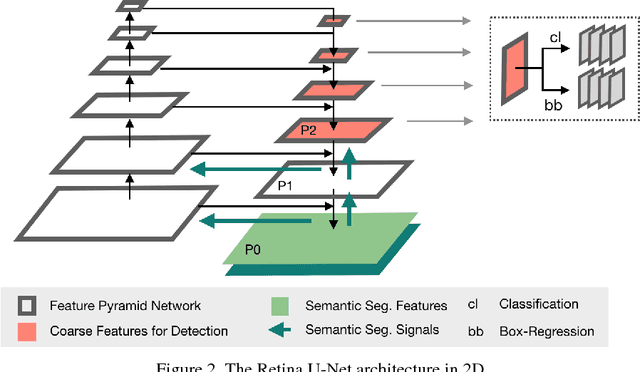
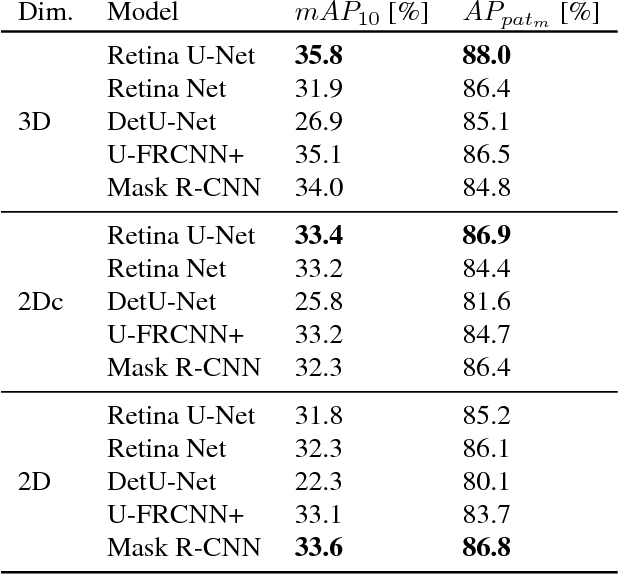
Abstract:The task of localizing and categorizing objects in medical images often remains formulated as a semantic segmentation problem. This approach, however, only indirectly solves the coarse localization task by predicting pixel-level scores, requiring ad-hoc heuristics when mapping back to object-level scores. State-of-the-art object detectors on the other hand, allow for individual object scoring in an end-to-end fashion, while ironically trading in the ability to exploit the full pixel-wise supervision signal. This can be particularly disadvantageous in the setting of medical image analysis, where data sets are notoriously small. In this paper, we propose Retina U-Net, a simple architecture, which naturally fuses the Retina Net one-stage detector with the U-Net architecture widely used for semantic segmentation in medical images. The proposed architecture recaptures discarded supervision signals by complementing object detection with an auxiliary task in the form of semantic segmentation without introducing the additional complexity of previously proposed two-stage detectors. We evaluate the importance of full segmentation supervision on two medical data sets, provide an in-depth analysis on a series of toy experiments and show how the corresponding performance gain grows in the limit of small data sets. Retina U-Net yields strong detection performance only reached by its more complex two-staged counterparts. Our framework including all methods implemented for operation on 2D and 3D images is available at github.com/pfjaeger/medicaldetectiontoolkit.
Domain Adaptation for Deviating Acquisition Protocols in CNN-based Lesion Classification on Diffusion-Weighted MR Images
Jul 17, 2018
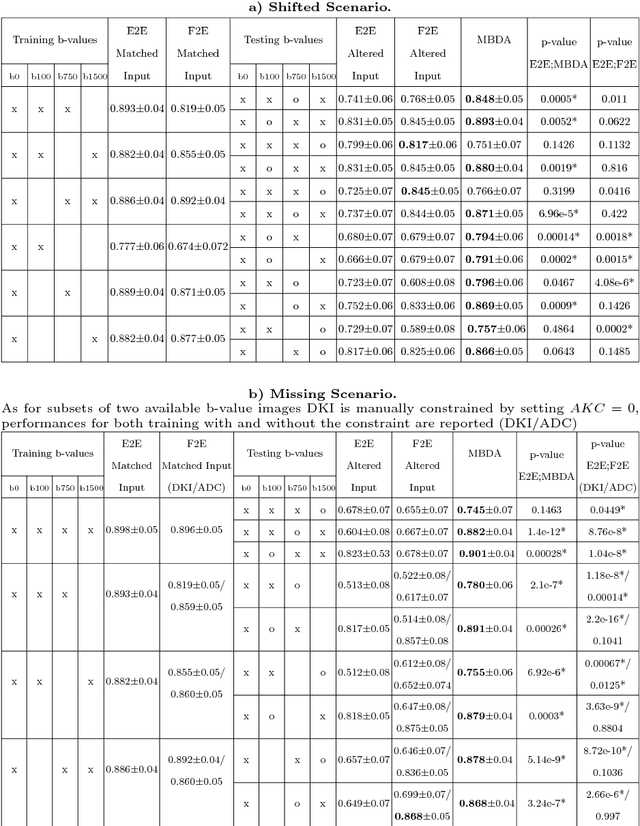
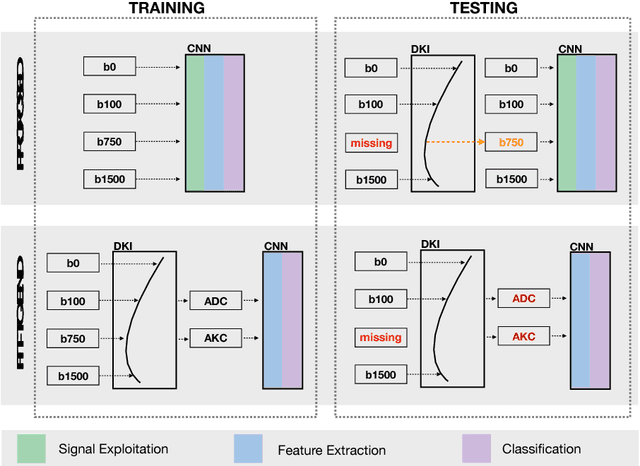
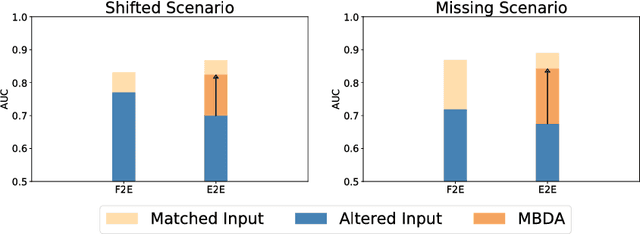
Abstract:End-to-end deep learning improves breast cancer classification on diffusion-weighted MR images (DWI) using a convolutional neural network (CNN) architecture. A limitation of CNN as opposed to previous model-based approaches is the dependence on specific DWI input channels used during training. However, in the context of large-scale application, methods agnostic towards heterogeneous inputs are desirable, due to the high deviation of scanning protocols between clinical sites. We propose model-based domain adaptation to overcome input dependencies and avoid re-training of networks at clinical sites by restoring training inputs from altered input channels given during deployment. We demonstrate the method's significant increase in classification performance and superiority over implicit domain adaptation provided by training-schemes operating on model-parameters instead of raw DWI images.
Revealing Hidden Potentials of the q-Space Signal in Breast Cancer
May 22, 2017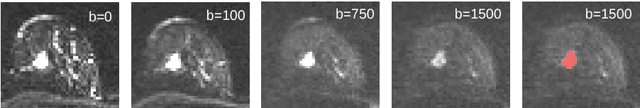

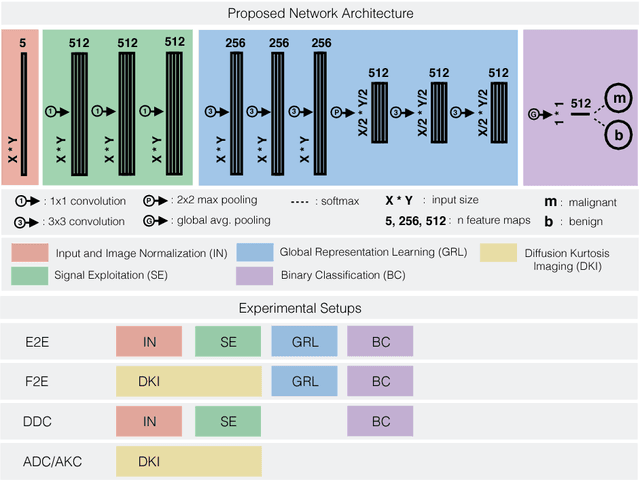
Abstract:Mammography screening for early detection of breast lesions currently suffers from high amounts of false positive findings, which result in unnecessary invasive biopsies. Diffusion-weighted MR images (DWI) can help to reduce many of these false-positive findings prior to biopsy. Current approaches estimate tissue properties by means of quantitative parameters taken from generative, biophysical models fit to the q-space encoded signal under certain assumptions regarding noise and spatial homogeneity. This process is prone to fitting instability and partial information loss due to model simplicity. We reveal unexplored potentials of the signal by integrating all data processing components into a convolutional neural network (CNN) architecture that is designed to propagate clinical target information down to the raw input images. This approach enables simultaneous and target-specific optimization of image normalization, signal exploitation, global representation learning and classification. Using a multicentric data set of 222 patients, we demonstrate that our approach significantly improves clinical decision making with respect to the current state of the art.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge