Fasil Gadjimuradov
Deep learning-guided weighted averaging for signal dropout compensation in diffusion-weighted imaging of the liver
Feb 20, 2022Abstract:Purpose: To develop an algorithm for the retrospective correction of signal dropout artifacts in abdominal diffusion-weighted imaging (DWI) resulting from cardiac motion. Methods: Given a set of image repetitions for a slice, a locally adaptive weighted averaging is proposed which aims to suppress the contribution of image regions affected by signal dropouts. Corresponding weight maps were estimated by a sliding-window algorithm which analyzed signal deviations from a patch-wise reference. In order to ensure the computation of a robust reference, repetitions were filtered by a classifier that was trained to detect images corrupted by signal dropouts. The proposed method, termed Deep Learning-guided Adaptive Weighted Averaging (DLAWA), was evaluated in terms of dropout suppression capability, bias reduction in the Apparent Diffusion Coefficient (ADC) and noise characteristics. Results: In the case of uniform averaging, motion-related dropouts caused signal attenuation and ADC overestimation in parts of the liver with the left lobe being affected particularly. Both effects could be substantially mitigated by DLAWA while preventing global penalties with respect to signal-to-noise ratio (SNR) due to local signal suppression. Performing evaluations on patient data, the capability to recover lesions concealed by signal dropouts was demonstrated as well. Further, DLAWA allowed for transparent control of the trade-off between SNR and signal dropout suppression by means of a few hyperparameters. Conclusion: This work presents an effective and flexible method for the local compensation of signal dropouts resulting from motion and pulsation. Since DLAWA follows a retrospective approach, no changes to the acquisition are required.
Towards Super-Resolution CEST MRI for Visualization of Small Structures
Dec 03, 2021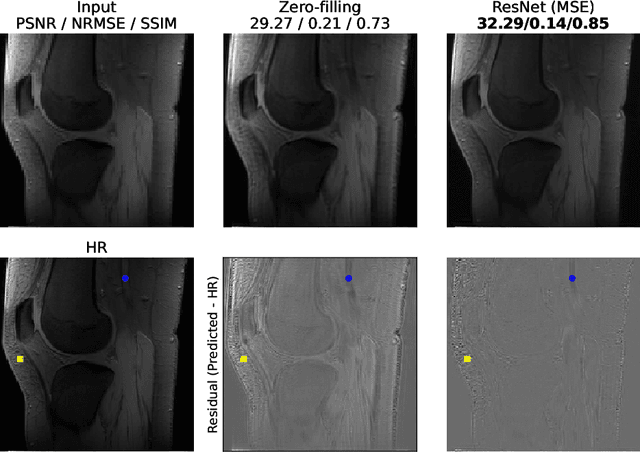
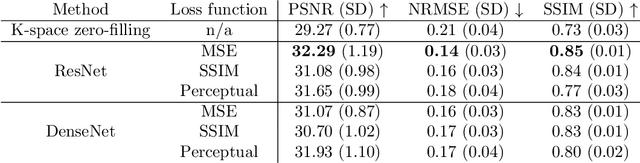
Abstract:The onset of rheumatic diseases such as rheumatoid arthritis is typically subclinical, which results in challenging early detection of the disease. However, characteristic changes in the anatomy can be detected using imaging techniques such as MRI or CT. Modern imaging techniques such as chemical exchange saturation transfer (CEST) MRI drive the hope to improve early detection even further through the imaging of metabolites in the body. To image small structures in the joints of patients, typically one of the first regions where changes due to the disease occur, a high resolution for the CEST MR imaging is necessary. Currently, however, CEST MR suffers from an inherently low resolution due to the underlying physical constraints of the acquisition. In this work we compared established up-sampling techniques to neural network-based super-resolution approaches. We could show, that neural networks are able to learn the mapping from low-resolution to high-resolution unsaturated CEST images considerably better than present methods. On the test set a PSNR of 32.29dB (+10%), a NRMSE of 0.14 (+28%), and a SSIM of 0.85 (+15%) could be achieved using a ResNet neural network, improving the baseline considerably. This work paves the way for the prospective investigation of neural networks for super-resolution CEST MRI and, followingly, might lead to a earlier detection of the onset of rheumatic diseases.
Robust partial Fourier reconstruction for diffusion-weighted imaging using a recurrent convolutional neural network
May 19, 2021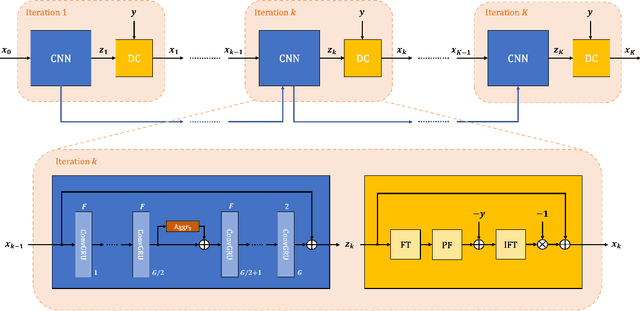
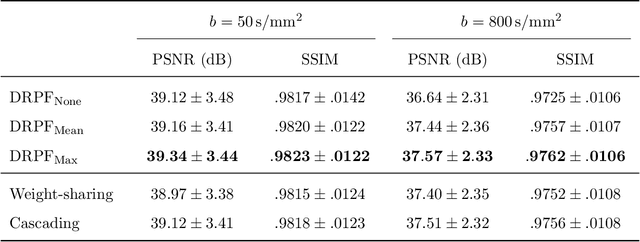
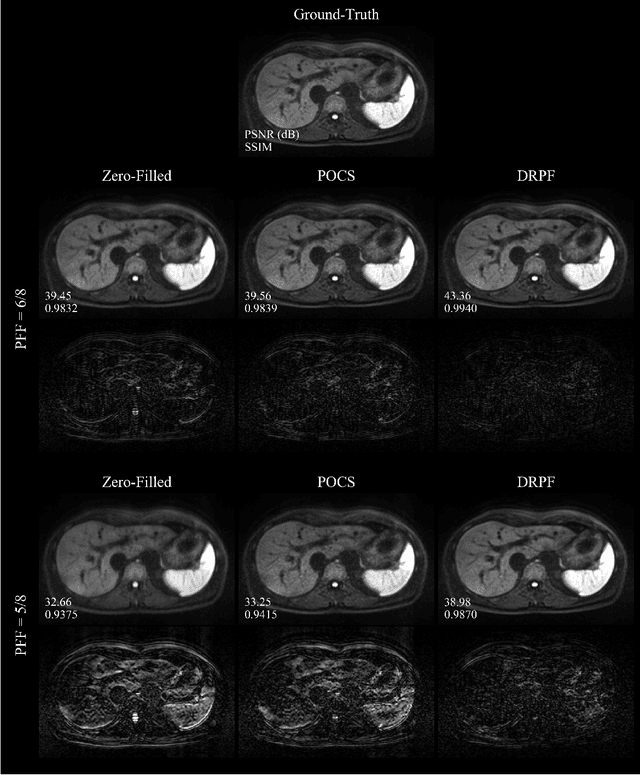
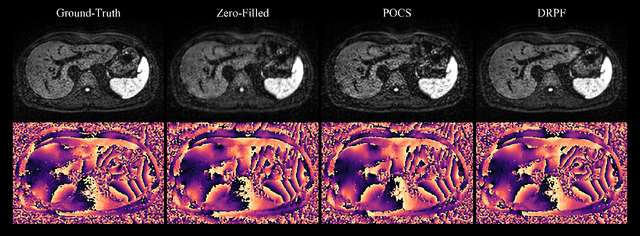
Abstract:Purpose: To develop an algorithm for robust partial Fourier (PF) reconstruction applicable to diffusion-weighted (DW) images with non-smooth phase variations. Methods: Based on an unrolled proximal splitting algorithm, a neural network architecture is derived which alternates between data consistency operations and regularization implemented by recurrent convolutions. In order to exploit correlations, multiple repetitions of the same slice are jointly reconstructed under consideration of permutation-equivariance. The proposed method is trained on DW liver data of 60 volunteers and evaluated on retrospectively and prospectively sub-sampled data of different anatomies and resolutions. In addition, the benefits of using a recurrent network over other unrolling strategies is investigated. Results: Conventional PF techniques can be significantly outperformed in terms of quantitative measures as well as perceptual image quality. The proposed method is able to generalize well to brain data with contrasts and resolution not present in the training set. The reduction in echo time (TE) associated with prospective PF-sampling enables DW imaging with higher signal. Also, the TE increase in acquisitions with higher resolution can be compensated for. It can be shown that unrolling by means of a recurrent network produced better results than using a weight-shared network or a cascade of networks. Conclusion: This work demonstrates that robust PF reconstruction of DW data is feasible even at strong PF factors in applications with severe phase variations. Since the proposed method does not rely on smoothness priors of the phase but uses learned recurrent convolutions instead, artifacts of conventional PF methods can be avoided.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge