Alan C. Evans
McConnell Brain Imaging Centre, Montreal Neurological Institute, McGill University, Montreal, QC, Canada
Analyzing Regional Organization of the Human Hippocampus in 3D-PLI Using Contrastive Learning and Geometric Unfolding
Feb 27, 2024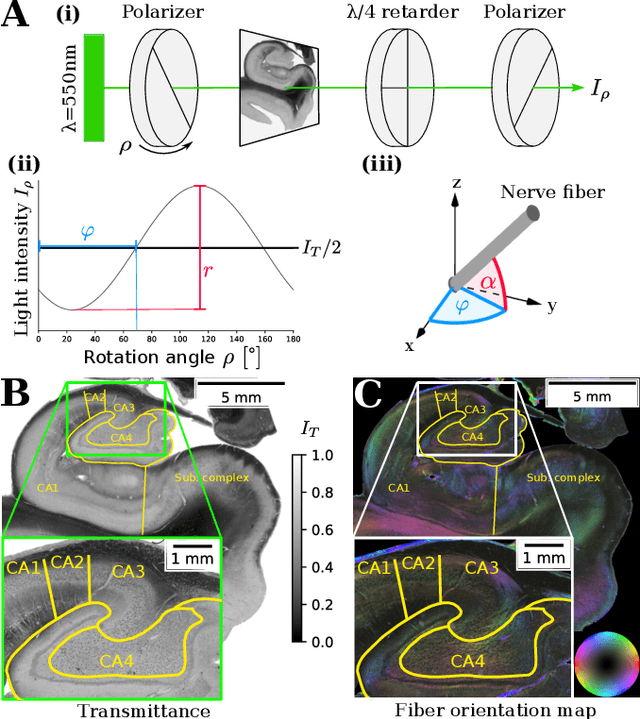
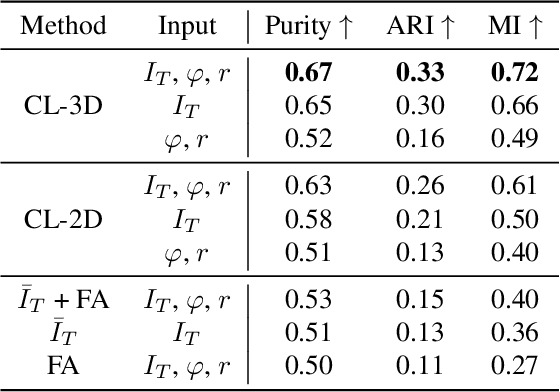
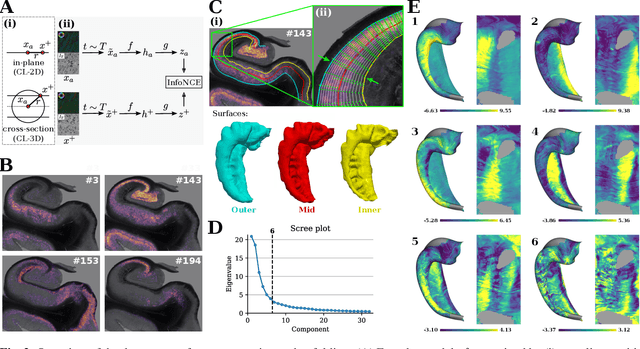
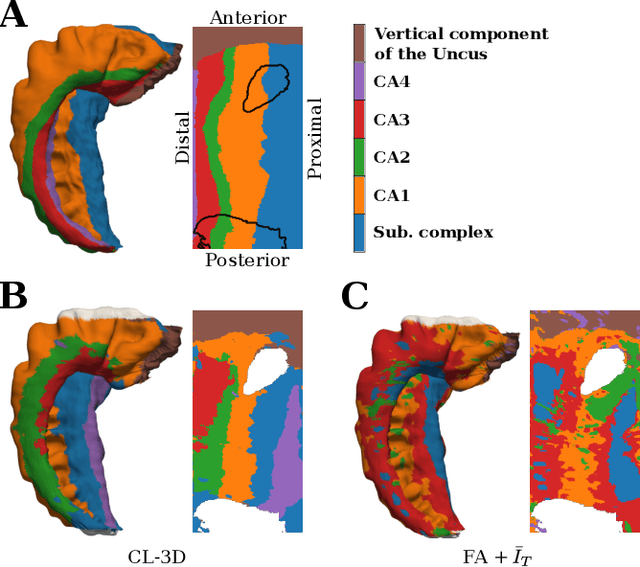
Abstract:Understanding the cortical organization of the human brain requires interpretable descriptors for distinct structural and functional imaging data. 3D polarized light imaging (3D-PLI) is an imaging modality for visualizing fiber architecture in postmortem brains with high resolution that also captures the presence of cell bodies, for example, to identify hippocampal subfields. The rich texture in 3D-PLI images, however, makes this modality particularly difficult to analyze and best practices for characterizing architectonic patterns still need to be established. In this work, we demonstrate a novel method to analyze the regional organization of the human hippocampus in 3D-PLI by combining recent advances in unfolding methods with deep texture features obtained using a self-supervised contrastive learning approach. We identify clusters in the representations that correspond well with classical descriptions of hippocampal subfields, lending validity to the developed methodology.
Data Augmentation Through Monte Carlo Arithmetic Leads to More Generalizable Classification in Connectomics
Sep 20, 2021Abstract:Machine learning models are commonly applied to human brain imaging datasets in an effort to associate function or structure with behaviour, health, or other individual phenotypes. Such models often rely on low-dimensional maps generated by complex processing pipelines. However, the numerical instabilities inherent to pipelines limit the fidelity of these maps and introduce computational bias. Monte Carlo Arithmetic, a technique for introducing controlled amounts of numerical noise, was used to perturb a structural connectome estimation pipeline, ultimately producing a range of plausible networks for each sample. The variability in the perturbed networks was captured in an augmented dataset, which was then used for an age classification task. We found that resampling brain networks across a series of such numerically perturbed outcomes led to improved performance in all tested classifiers, preprocessing strategies, and dimensionality reduction techniques. Importantly, we find that this benefit does not hinge on a large number of perturbations, suggesting that even minimally perturbing a dataset adds meaningful variance which can be captured in the subsequently designed models.
Convolutional Neural Networks for cytoarchitectonic brain mapping at large scale
Nov 25, 2020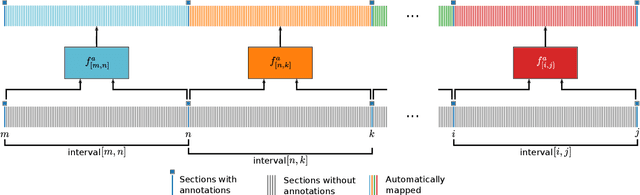

Abstract:Human brain atlases provide spatial reference systems for data characterizing brain organization at different levels, coming from different brains. Cytoarchitecture is a basic principle of the microstructural organization of the brain, as regional differences in the arrangement and composition of neuronal cells are indicators of changes in connectivity and function. Automated scanning procedures and observer-independent methods are prerequisites to reliably identify cytoarchitectonic areas, and to achieve reproducible models of brain segregation. Time becomes a key factor when moving from the analysis of single regions of interest towards high-throughput scanning of large series of whole-brain sections. Here we present a new workflow for mapping cytoarchitectonic areas in large series of cell-body stained histological sections of human postmortem brains. It is based on a Deep Convolutional Neural Network (CNN), which is trained on a pair of section images with annotations, with a large number of un-annotated sections in between. The model learns to create all missing annotations in between with high accuracy, and faster than our previous workflow based on observer-independent mapping. The new workflow does not require preceding 3D-reconstruction of sections, and is robust against histological artefacts. It processes large data sets with sizes in the order of multiple Terabytes efficiently. The workflow was integrated into a web interface, to allow access without expertise in deep learning and batch computing. Applying deep neural networks for cytoarchitectonic mapping opens new perspectives to enable high-resolution models of brain areas, introducing CNNs to identify borders of brain areas.
Local semi-supervised approach to brain tissue classification in child brain MRI
May 20, 2020
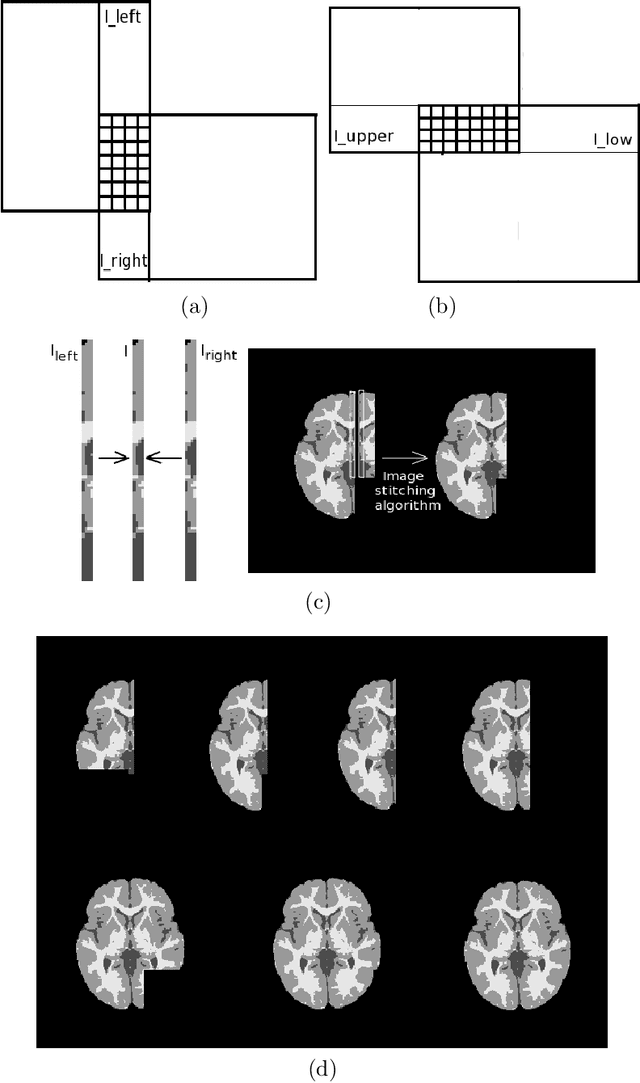
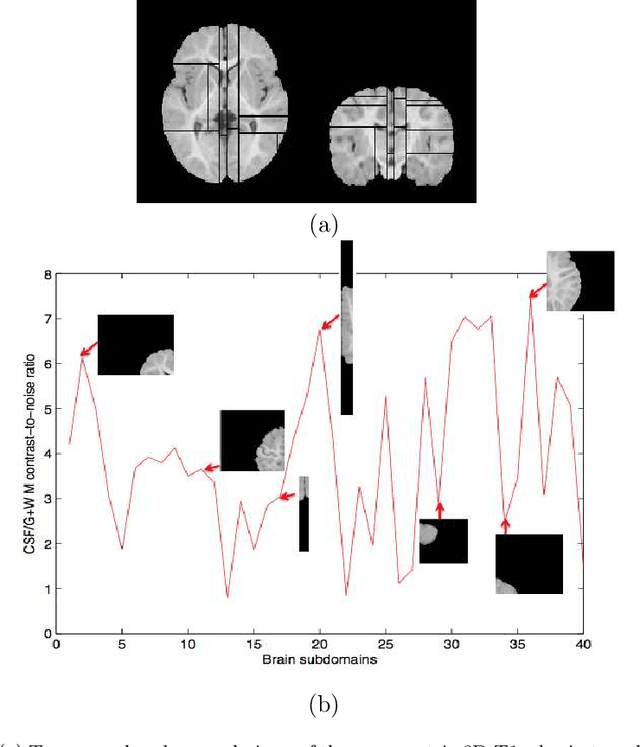
Abstract:Most segmentation methods in child brain MRI are supervised and are based on global intensity distributions of major brain structures. The successful implementation of a supervised approach depends on availability of an age-appropriate probabilistic brain atlas. For the study of early normal brain development, the construction of such a brain atlas remains a significant challenge. Moreover, using global intensity statistics leads to inaccurate detection of major brain tissue classes due to substantial intensity variations of MR signal within the constituent parts of early developing brain. In order to overcome these methodological limitations we develop a local, semi-supervised framework. It is based on Kernel Fisher Discriminant Analysis (KFDA) for pattern recognition, combined with an objective structural similarity index (SSIM) for perceptual image quality assessment. The proposed method performs optimal brain partitioning into subdomains having different average intensity values followed by SSIM-guided computation of separating surfaces between the constituent brain parts. The classified image subdomains are then stitched slice by slice via simulated annealing to form a global image of the classified brain. In this paper, we consider classification into major tissue classes (white matter and grey matter) and the cerebrospinal fluid and illustrate the proposed framework on examples of brain templates for ages 8 to 11 months and ages 44 to 60 months. We show that our method improves detection of the tissue classes by its comparison to state-of-the-art classification techniques known as Partial Volume Estimation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge