Christian Schiffer
From Fibers to Cells: Fourier-Based Registration Enables Virtual Cresyl Violet Staining From 3D Polarized Light Imaging
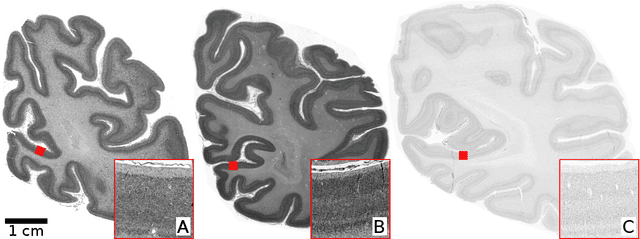
May 16, 2025Abstract:Comprehensive assessment of the various aspects of the brain's microstructure requires the use of complementary imaging techniques. This includes measuring the spatial distribution of cell bodies (cytoarchitecture) and nerve fibers (myeloarchitecture). The gold standard for cytoarchitectonic analysis is light microscopic imaging of cell-body stained tissue sections. To reveal the 3D orientations of nerve fibers, 3D Polarized Light Imaging (3D-PLI) has been introduced as a reliable technique providing a resolution in the micrometer range while allowing processing of series of complete brain sections. 3D-PLI acquisition is label-free and allows subsequent staining of sections after measurement. By post-staining for cell bodies, a direct link between fiber- and cytoarchitecture can potentially be established within the same section. However, inevitable distortions introduced during the staining process make a nonlinear and cross-modal registration necessary in order to study the detailed relationships between cells and fibers in the images. In addition, the complexity of processing histological sections for post-staining only allows for a limited number of samples. In this work, we take advantage of deep learning methods for image-to-image translation to generate a virtual staining of 3D-PLI that is spatially aligned at the cellular level. In a supervised setting, we build on a unique dataset of brain sections, to which Cresyl violet staining has been applied after 3D-PLI measurement. To ensure high correspondence between both modalities, we address the misalignment of training data using Fourier-based registration methods. In this way, registration can be efficiently calculated during training for local image patches of target and predicted staining. We demonstrate that the proposed method enables prediction of a Cresyl violet staining from 3D-PLI, matching individual cell instances.
Self-Supervised Representation Learning for Nerve Fiber Distribution Patterns in 3D-PLI
Jan 30, 2024Abstract:A comprehensive understanding of the organizational principles in the human brain requires, among other factors, well-quantifiable descriptors of nerve fiber architecture. Three-dimensional polarized light imaging (3D-PLI) is a microscopic imaging technique that enables insights into the fine-grained organization of myelinated nerve fibers with high resolution. Descriptors characterizing the fiber architecture observed in 3D-PLI would enable downstream analysis tasks such as multimodal correlation studies, clustering, and mapping. However, best practices for observer-independent characterization of fiber architecture in 3D-PLI are not yet available. To this end, we propose the application of a fully data-driven approach to characterize nerve fiber architecture in 3D-PLI images using self-supervised representation learning. We introduce a 3D-Context Contrastive Learning (CL-3D) objective that utilizes the spatial neighborhood of texture examples across histological brain sections of a 3D reconstructed volume to sample positive pairs for contrastive learning. We combine this sampling strategy with specifically designed image augmentations to gain robustness to typical variations in 3D-PLI parameter maps. The approach is demonstrated for the 3D reconstructed occipital lobe of a vervet monkey brain. We show that extracted features are highly sensitive to different configurations of nerve fibers, yet robust to variations between consecutive brain sections arising from histological processing. We demonstrate their practical applicability for retrieving clusters of homogeneous fiber architecture and performing data mining for interactively selected templates of specific components of fiber architecture such as U-fibers.
Denoising Diffusion Probabilistic Models for Image Inpainting of Cell Distributions in the Human Brain
Nov 28, 2023



Abstract:Recent advances in imaging and high-performance computing have made it possible to image the entire human brain at the cellular level. This is the basis to study the multi-scale architecture of the brain regarding its subdivision into brain areas and nuclei, cortical layers, columns, and cell clusters down to single cell morphology Methods for brain mapping and cell segmentation exploit such images to enable rapid and automated analysis of cytoarchitecture and cell distribution in complete series of histological sections. However, the presence of inevitable processing artifacts in the image data caused by missing sections, tears in the tissue, or staining variations remains the primary reason for gaps in the resulting image data. To this end we aim to provide a model that can fill in missing information in a reliable way, following the true cell distribution at different scales. Inspired by the recent success in image generation, we propose a denoising diffusion probabilistic model (DDPM), trained on light-microscopic scans of cell-body stained sections. We extend this model with the RePaint method to impute missing or replace corrupted image data. We show that our trained DDPM is able to generate highly realistic image information for this purpose, generating plausible cell statistics and cytoarchitectonic patterns. We validate its outputs using two established downstream task models trained on the same data.
2D histology meets 3D topology: Cytoarchitectonic brain mapping with Graph Neural Networks
Mar 09, 2021



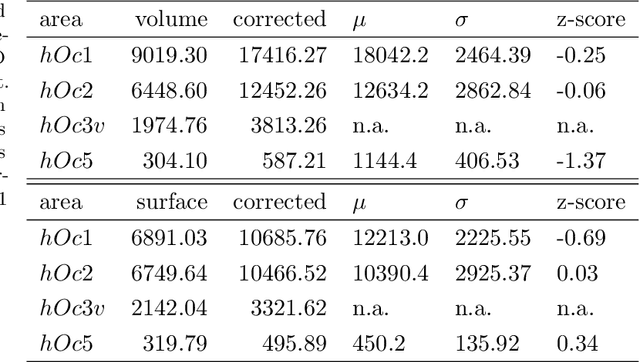
Abstract:Cytoarchitecture describes the spatial organization of neuronal cells in the brain, including their arrangement into layers and columns with respect to cell density, orientation, or presence of certain cell types. It allows to segregate the brain into cortical areas and subcortical nuclei, links structure with connectivity and function, and provides a microstructural reference for human brain atlases. Mapping boundaries between areas requires to scan histological sections at microscopic resolution. While recent high-throughput scanners allow to scan a complete human brain in the order of a year, it is practically impossible to delineate regions at the same pace using the established gold standard method. Researchers have recently addressed cytoarchitectonic mapping of cortical regions with deep neural networks, relying on image patches from individual 2D sections for classification. However, the 3D context, which is needed to disambiguate complex or obliquely cut brain regions, is not taken into account. In this work, we combine 2D histology with 3D topology by reformulating the mapping task as a node classification problem on an approximate 3D midsurface mesh through the isocortex. We extract deep features from cortical patches in 2D histological sections which are descriptive of cytoarchitecture, and assign them to the corresponding nodes on the 3D mesh to construct a large attributed graph. By solving the brain mapping problem on this graph using graph neural networks, we obtain significantly improved classification results. The proposed framework lends itself nicely to integration of additional neuroanatomical priors for mapping.
Contrastive Representation Learning for Whole Brain Cytoarchitectonic Mapping in Histological Human Brain Sections
Nov 25, 2020



Abstract:Cytoarchitectonic maps provide microstructural reference parcellations of the brain, describing its organization in terms of the spatial arrangement of neuronal cell bodies as measured from histological tissue sections. Recent work provided the first automatic segmentations of cytoarchitectonic areas in the visual system using Convolutional Neural Networks. We aim to extend this approach to become applicable to a wider range of brain areas, envisioning a solution for mapping the complete human brain. Inspired by recent success in image classification, we propose a contrastive learning objective for encoding microscopic image patches into robust microstructural features, which are efficient for cytoarchitectonic area classification. We show that a model pre-trained using this learning task outperforms a model trained from scratch, as well as a model pre-trained on a recently proposed auxiliary task. We perform cluster analysis in the feature space to show that the learned representations form anatomically meaningful groups.
Convolutional Neural Networks for cytoarchitectonic brain mapping at large scale
Nov 25, 2020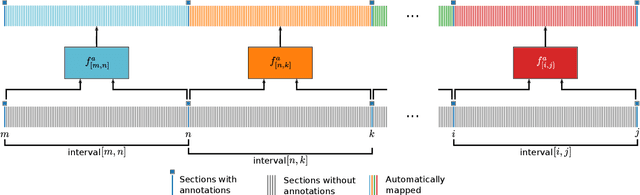

Abstract:Human brain atlases provide spatial reference systems for data characterizing brain organization at different levels, coming from different brains. Cytoarchitecture is a basic principle of the microstructural organization of the brain, as regional differences in the arrangement and composition of neuronal cells are indicators of changes in connectivity and function. Automated scanning procedures and observer-independent methods are prerequisites to reliably identify cytoarchitectonic areas, and to achieve reproducible models of brain segregation. Time becomes a key factor when moving from the analysis of single regions of interest towards high-throughput scanning of large series of whole-brain sections. Here we present a new workflow for mapping cytoarchitectonic areas in large series of cell-body stained histological sections of human postmortem brains. It is based on a Deep Convolutional Neural Network (CNN), which is trained on a pair of section images with annotations, with a large number of un-annotated sections in between. The model learns to create all missing annotations in between with high accuracy, and faster than our previous workflow based on observer-independent mapping. The new workflow does not require preceding 3D-reconstruction of sections, and is robust against histological artefacts. It processes large data sets with sizes in the order of multiple Terabytes efficiently. The workflow was integrated into a web interface, to allow access without expertise in deep learning and batch computing. Applying deep neural networks for cytoarchitectonic mapping opens new perspectives to enable high-resolution models of brain areas, introducing CNNs to identify borders of brain areas.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge