Hannah Spitzer
Robust and Generalisable Segmentation of Subtle Epilepsy-causing Lesions: a Graph Convolutional Approach
Jun 05, 2023



Abstract:Focal cortical dysplasia (FCD) is a leading cause of drug-resistant focal epilepsy, which can be cured by surgery. These lesions are extremely subtle and often missed even by expert neuroradiologists. "Ground truth" manual lesion masks are therefore expensive, limited and have large inter-rater variability. Existing FCD detection methods are limited by high numbers of false positive predictions, primarily due to vertex- or patch-based approaches that lack whole-brain context. Here, we propose to approach the problem as semantic segmentation using graph convolutional networks (GCN), which allows our model to learn spatial relationships between brain regions. To address the specific challenges of FCD identification, our proposed model includes an auxiliary loss to predict distance from the lesion to reduce false positives and a weak supervision classification loss to facilitate learning from uncertain lesion masks. On a multi-centre dataset of 1015 participants with surface-based features and manual lesion masks from structural MRI data, the proposed GCN achieved an AUC of 0.74, a significant improvement against a previously used vertex-wise multi-layer perceptron (MLP) classifier (AUC 0.64). With sensitivity thresholded at 67%, the GCN had a specificity of 71% in comparison to 49% when using the MLP. This improvement in specificity is vital for clinical integration of lesion-detection tools into the radiological workflow, through increasing clinical confidence in the use of AI radiological adjuncts and reducing the number of areas requiring expert review.
Noise transfer for unsupervised domain adaptation of retinal OCT images
Sep 16, 2022Abstract:Optical coherence tomography (OCT) imaging from different camera devices causes challenging domain shifts and can cause a severe drop in accuracy for machine learning models. In this work, we introduce a minimal noise adaptation method based on a singular value decomposition (SVDNA) to overcome the domain gap between target domains from three different device manufacturers in retinal OCT imaging. Our method utilizes the difference in noise structure to successfully bridge the domain gap between different OCT devices and transfer the style from unlabeled target domain images to source images for which manual annotations are available. We demonstrate how this method, despite its simplicity, compares or even outperforms state-of-the-art unsupervised domain adaptation methods for semantic segmentation on a public OCT dataset. SVDNA can be integrated with just a few lines of code into the augmentation pipeline of any network which is in contrast to many state-of-the-art domain adaptation methods which often need to change the underlying model architecture or train a separate style transfer model. The full code implementation for SVDNA is available at https://github.com/ValentinKoch/SVDNA.
Convolutional Neural Networks for cytoarchitectonic brain mapping at large scale
Nov 25, 2020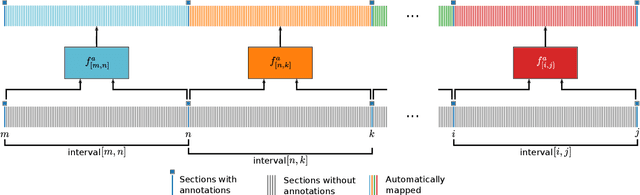

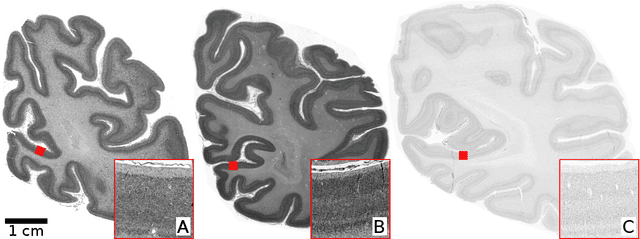
Abstract:Human brain atlases provide spatial reference systems for data characterizing brain organization at different levels, coming from different brains. Cytoarchitecture is a basic principle of the microstructural organization of the brain, as regional differences in the arrangement and composition of neuronal cells are indicators of changes in connectivity and function. Automated scanning procedures and observer-independent methods are prerequisites to reliably identify cytoarchitectonic areas, and to achieve reproducible models of brain segregation. Time becomes a key factor when moving from the analysis of single regions of interest towards high-throughput scanning of large series of whole-brain sections. Here we present a new workflow for mapping cytoarchitectonic areas in large series of cell-body stained histological sections of human postmortem brains. It is based on a Deep Convolutional Neural Network (CNN), which is trained on a pair of section images with annotations, with a large number of un-annotated sections in between. The model learns to create all missing annotations in between with high accuracy, and faster than our previous workflow based on observer-independent mapping. The new workflow does not require preceding 3D-reconstruction of sections, and is robust against histological artefacts. It processes large data sets with sizes in the order of multiple Terabytes efficiently. The workflow was integrated into a web interface, to allow access without expertise in deep learning and batch computing. Applying deep neural networks for cytoarchitectonic mapping opens new perspectives to enable high-resolution models of brain areas, introducing CNNs to identify borders of brain areas.
Improving Cytoarchitectonic Segmentation of Human Brain Areas with Self-supervised Siamese Networks
Jun 13, 2018
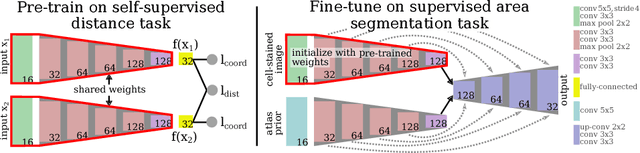
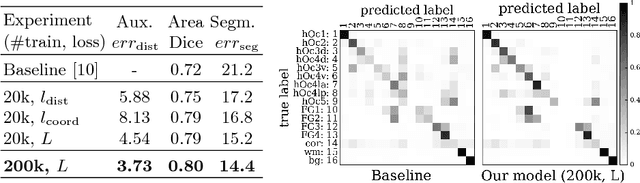
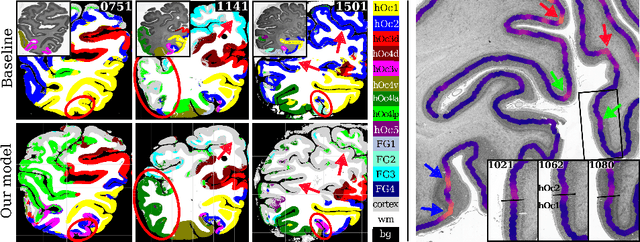
Abstract:Cytoarchitectonic parcellations of the human brain serve as anatomical references in multimodal atlas frameworks. They are based on analysis of cell-body stained histological sections and the identification of borders between brain areas. The de-facto standard involves a semi-automatic, reproducible border detection, but does not scale with high-throughput imaging in large series of sections at microscopical resolution. Automatic parcellation, however, is extremely challenging due to high variation in the data, and the need for a large field of view at microscopic resolution. The performance of a recently proposed Convolutional Neural Network model that addresses this problem especially suffers from the naturally limited amount of expert annotations for training. To circumvent this limitation, we propose to pre-train neural networks on a self-supervised auxiliary task, predicting the 3D distance between two patches sampled from the same brain. Compared to a random initialization, fine-tuning from these networks results in significantly better segmentations. We show that the self-supervised model has implicitly learned to distinguish several cortical brain areas -- a strong indicator that the proposed auxiliary task is appropriate for cytoarchitectonic mapping.
Parcellation of Visual Cortex on high-resolution histological Brain Sections using Convolutional Neural Networks
May 30, 2017



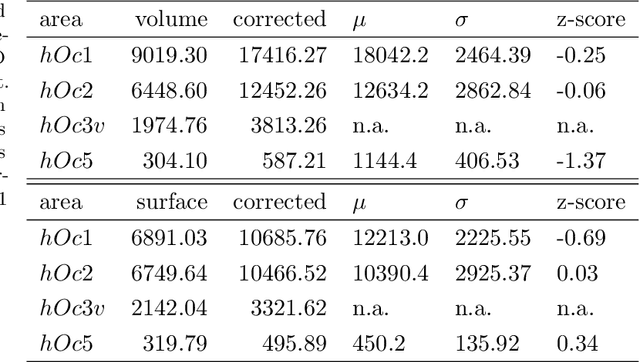
Abstract:Microscopic analysis of histological sections is considered the "gold standard" to verify structural parcellations in the human brain. Its high resolution allows the study of laminar and columnar patterns of cell distributions, which build an important basis for the simulation of cortical areas and networks. However, such cytoarchitectonic mapping is a semiautomatic, time consuming process that does not scale with high throughput imaging. We present an automatic approach for parcellating histological sections at 2um resolution. It is based on a convolutional neural network that combines topological information from probabilistic atlases with the texture features learned from high-resolution cell-body stained images. The model is applied to visual areas and trained on a sparse set of partial annotations. We show how predictions are transferable to new brains and spatially consistent across sections.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge