Kai Kiwitz
Convolutional Neural Networks for cytoarchitectonic brain mapping at large scale
Nov 25, 2020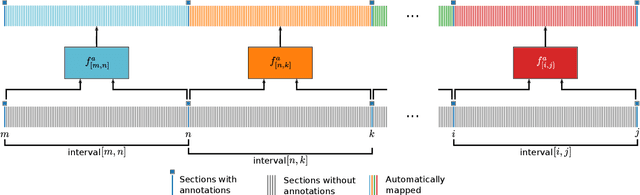

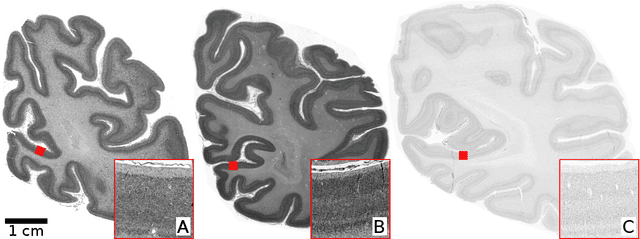
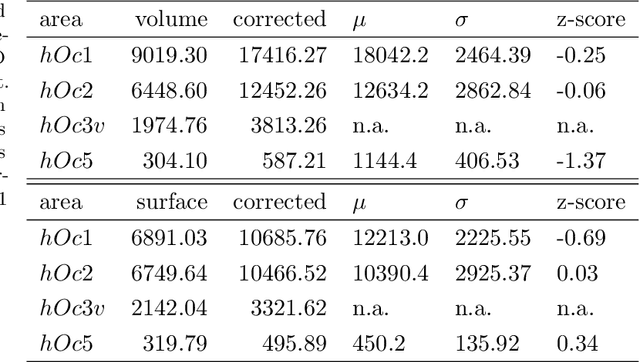
Abstract:Human brain atlases provide spatial reference systems for data characterizing brain organization at different levels, coming from different brains. Cytoarchitecture is a basic principle of the microstructural organization of the brain, as regional differences in the arrangement and composition of neuronal cells are indicators of changes in connectivity and function. Automated scanning procedures and observer-independent methods are prerequisites to reliably identify cytoarchitectonic areas, and to achieve reproducible models of brain segregation. Time becomes a key factor when moving from the analysis of single regions of interest towards high-throughput scanning of large series of whole-brain sections. Here we present a new workflow for mapping cytoarchitectonic areas in large series of cell-body stained histological sections of human postmortem brains. It is based on a Deep Convolutional Neural Network (CNN), which is trained on a pair of section images with annotations, with a large number of un-annotated sections in between. The model learns to create all missing annotations in between with high accuracy, and faster than our previous workflow based on observer-independent mapping. The new workflow does not require preceding 3D-reconstruction of sections, and is robust against histological artefacts. It processes large data sets with sizes in the order of multiple Terabytes efficiently. The workflow was integrated into a web interface, to allow access without expertise in deep learning and batch computing. Applying deep neural networks for cytoarchitectonic mapping opens new perspectives to enable high-resolution models of brain areas, introducing CNNs to identify borders of brain areas.
Improving Cytoarchitectonic Segmentation of Human Brain Areas with Self-supervised Siamese Networks
Jun 13, 2018
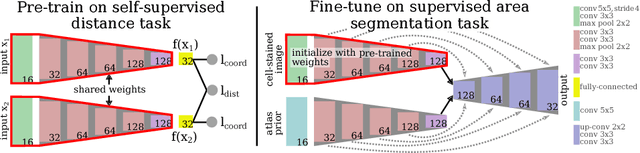
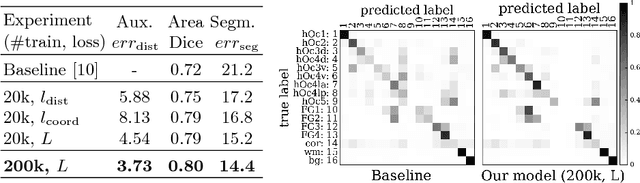
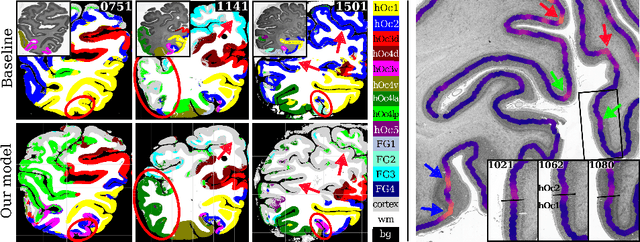
Abstract:Cytoarchitectonic parcellations of the human brain serve as anatomical references in multimodal atlas frameworks. They are based on analysis of cell-body stained histological sections and the identification of borders between brain areas. The de-facto standard involves a semi-automatic, reproducible border detection, but does not scale with high-throughput imaging in large series of sections at microscopical resolution. Automatic parcellation, however, is extremely challenging due to high variation in the data, and the need for a large field of view at microscopic resolution. The performance of a recently proposed Convolutional Neural Network model that addresses this problem especially suffers from the naturally limited amount of expert annotations for training. To circumvent this limitation, we propose to pre-train neural networks on a self-supervised auxiliary task, predicting the 3D distance between two patches sampled from the same brain. Compared to a random initialization, fine-tuning from these networks results in significantly better segmentations. We show that the self-supervised model has implicitly learned to distinguish several cortical brain areas -- a strong indicator that the proposed auxiliary task is appropriate for cytoarchitectonic mapping.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge